Interleukin 17C: Is It A Host Defense Factor In The Oral Innate Immunity?- Juniper Publishers
Juniper Publishers- Open Access Journal of Dentistry & Oral Health
Abstract
Toll-like receptors (TLRs) expressed on oral epithelial cells (OECs) enable rapid response to microorganisms by secreting antimicrobial peptides as well as cytokines that play pivotal roles in antimicrobial immunity. Several lines of investigation have revealed that cytokines play important roles not only in tissue homeostasis, but also in the pathogenesis of infectious disease including periodontitis. OECs also express receptors for a number of different cytokines. Thus, cytokines secreted by OECs may play an autocrine role or may influence adjacent non-epithelial cells. IL-17C, a member of the IL-17 cytokine family, is mainly produced by epithelial cells. Its role in the gut- immune system has recently been proposed, however, the biological significance of IL-17C and its receptor on OECs in the maintenance of oral mucosal homeostasis and pathogenesis of periodontitis has not been systematically investigated. This mini review will give a current understanding of immunomodulatory roles of IL-17C to maintain mucosal homeostasis, including oral cavity.
Keywords: Interleukin-17C; β-defensin; Oral mucosa; Innate immunity
Introduction
The oral cavity harbors a diverse and complex microbial community. The oral microbial flora contains approximately 500 species, and is composed of both commensal and pathogenic species [1]. Bacteria accumulate on both the hard and soft oral tissues in a sessile biofilm. In this highly antigenic environment, oral mucosal tissue must maintain tolerance to commensal bacteria and other molecules, such as proteins in food. Under certain circumstances, however, the oral bacterial flora can induce an immune response resulting in inflammatory manifestations, such as periodontitis.
Oral innate immunity
The mucosal surface of the oral cavity is protected primarily by secretory components of the salivary glands and oral epithelium. Saliva contains an array of molecules that play vital roles in the innate and adaptive host defense mechanisms [2]. Innate host defense factors in saliva function to promote bacterial agglutination, inhibit bacterial growth, inhibit bacterial proteases, or prevent bacteria from cell wall synthesis. Secretory IgA is the major antibody in saliva and specifically binds to target antigens.
The oral epithelium functions as the first line of defense against invading microorganisms to maintain oral mucosal homeostasis. Toll-like receptors (TLRs) expressed on oral epithelial cells (OECs) enable rapid response to microorganisms by secreting chemokines, cytokines and antimicrobial peptides that play pivotal roles in antimicrobial immunity [3]. For example, ligation of TLR9 by CpG induced IL-8 expression in gingival epithelial cells [4]. IL-8 is a chemoattractant for neutrophils, recruiting them to the site of infection. OECs also express receptors for a number of different cytokines. Thus, cytokines secreted by OECs may play an autocrine role or may influence adjacent non-epithelial cells. In addition, cytokines and other molecules in saliva [5] may stimulate OECs to secrete cytokines, chemokines, and antimicrobial peptides. Periodontal disease is a type of chronic, destructive, inflammatory disease resulting from a polymicrobial disruption of the oral mucosal homeostasis [6].
The β-defensins are antimicrobial peptides that possess a broad spectrum of activity against both Gram-negative and Gram-positive bacteria as well as some fungi and viruses [7,8]. In addition to their direct antimicrobial activity, human β-defensins (hBDs) also directly stimulate antigen- presenting dendritic cells and memory T cells, and thus can link innate and adaptive immune responses [3,9-11]. Human β-defensin 1 (hBD1) is constitutively expressed by OECs, while the expression of hBD2 and hBD3 is inducible. Epithelial cells produce hBD2 and hBD3 following stimulation with microorganisms (Gram-negative, Gram-positive bacteria and Candida albicans) or cytokines such as TNF-α and IL-1β [3,12-14].
IL-17C
IL-17C, a member of the IL-17 cytokine family, is mainly produced by epithelial cells [15,16]. Colon and tracheal epithelial cells as well as epidermal keratinocytes revealed enhanced secretion of IL-17C in response to heat-killed Escherichia coli. Moreover, TLR2 and TLR5 ligands, and cytokines, such as TNF-α and IL-1β, also regulate the expression of IL-17C in colon epithelial cells and epidermal keratinocytes, respectively [16]. IL- 17C binds to the IL-17RA/IL-17RE receptor complex expressed on the epithelial cells, resulting in the recruitment of adapter molecule, Act1, to the receptor complex. This triggers the NFκB activation, leading to the expression of target gene that plays a role in the innate host defense mechanism [15-17]. IL-17C also plays a role in T cell polarization. IL-17C binding to IL-17RE directs the differentiation of CD4+ T cells into Th17 populations [18].
recombinant human (rh) IL-17C enhanced hBD2 secretion [16]. Mice lacking IL-17C revealed exacerbated dextran sodium sulfate (DSS) induced colitis associated with degraded expression of tight junction protein in colonic epithelial cells [17]. On the contrary, mice lacking IL-17C were partially resistant to experimental autoimmune encephalomyelitis (EAE) [18], indicating that IL-17C is a key molecule for the pathogenesis of EAE. This can be explained, at least in part, by the difference in the cell type predominates in the disease. DSS-induced colitis is a disease model of innate immunity [19], while EAE is Th17 mediated condition [18]. Taken together, IL-17C can be either anti- or pro-inflammator cytokine. IL-17C exerts its anti-inflammatory function in the innate immunity. It functions as pro- inflammatory cytokine in the adaptive immunity. However, the biological significance of IL-17C and its receptor on OECs in maintaining oral mucosal homeostasis and pathogenesis of periodontitis has not been systematically investigated.
Hypothetical model of IL-17C in oral innate immunity
Intestinal homeostasis mediated by IL-17C has been wellstudied. However, the biological significance of IL-17C and its receptor on OECs in maintaining oral mucosal homeostasis and pathogenesis of periodontitis has not been systematically investigated. Recent study showed the slight immunoreactivity against IL-17C in healthy oral epithelium [20]. In addition, OECs expressed IL-17C, IL-17RA, and IL-17RE mRNA. Our preliminary data showed that IL-17C was secreted by OECs in response to TLR ligands. These data suggests that IL-17C is constitutively expressed by OECs and bacterial challenge enhances its expression. Since IL- 17C induces hBD2 from keratinocytes, we hypothesize that IL-17C binds back to OECs in autocrine and/or paracrine manner, leading to hBD2 secretion from these cells (Figure 1).
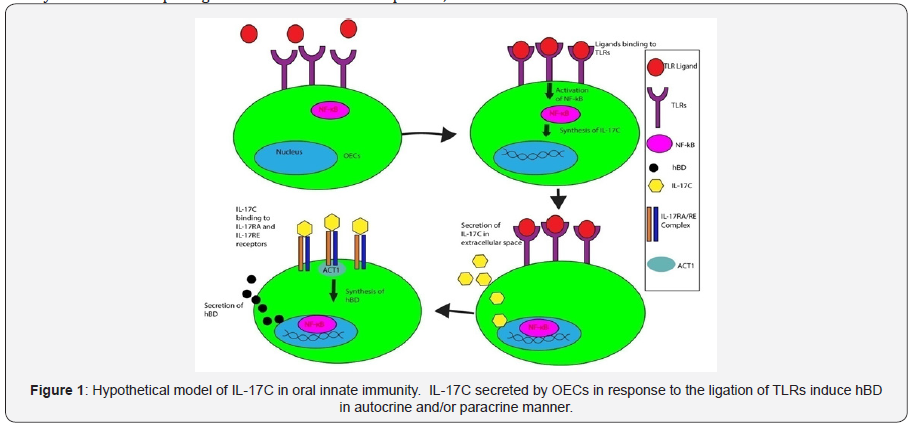
Conclusion
IL-17C is one of the key regulatory molecules to maintain homeostasis of intestinal mucosa and we proposed its role in oral innate immunity. The experiments have been underway to investigate whether or not IL-17C secreted by OECs play a role in maintaining oral mucosal homeostasis.
Acknowledgement
This work was supported in part by a Research Pilot Project Award 03-Activity 092 (T. Chino) from University of the Pacific, Arthur A. Dugoni School of Dentistry,
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/aboutus.php


Comments
Post a Comment