Identification of Mercury Levels on the Operator’s FaceMask During the Removal Of Dental Amalgamas: Pilot Study- Juniper Publishers
Juniper Publishers-Open Access Journal of Dentistry & Oral Health
Abstract
Several cases of mercury poisoning and embryotoxicity among dental personnel have been documented. The exposure level of mercury vapor upon removal of an amalgam restoration, without water irrigation and without ventilation is 0.19 mg/m3. Dentists exposed to 16.7μg/m3 of mercury in the air showed deficiencies in logic and vision. Facemasks have been used to reduce workers’ exposure to infectious and toxic aerosol material.
Objective: Several cases of mercury poisoning and embryotoxicity among dental personnel have been documented. The exposure level of mercury vapor upon removal of an amalgam restoration, without water irrigation and without ventilation is 0.19 mg/m3. Dentists exposed to 16.7μg/m3 of mercury in the air showed deficiencies in logic and vision. Facemasks have been used to reduce workers’ exposure to infectious and toxic aerosol material.
Methods and Materials:Several cases of mercury poisoning and embryotoxicity among dental personnel have been documented. The exposure level of mercury vapor upon removal of an amalgam restoration, without water irrigation and without ventilation is 0.19 mg/m3. Dentists exposed to 16.7μg/m3 of mercury in the air showed deficiencies in logic and vision. Facemasks have been used to reduce workers’ exposure to infectious and toxic aerosol material.
Results: Several cases of mercury poisoning and embryotoxicity among dental personnel have been documented. The exposure level of mercury vapor upon removal of an amalgam restoration, without water irrigation and without ventilation is 0.19 mg/m3. Dentists exposed to 16.7μg/m3 of mercury in the air showed deficiencies in logic and vision. Facemasks have been used to reduce workers’ exposure to infectious and toxic aerosol material.
Conclusion: Several cases of mercury poisoning and embryotoxicity among dental personnel have been documented. The exposure level of mercury vapor upon removal of an amalgam restoration, without water irrigation and without ventilation is 0.19 mg/m3. Dentists exposed to 16.7μg/m3 of mercury in the air showed deficiencies in logic and vision. Facemasks have been used to reduce workers’ exposure to infectious and toxic aerosol material.
Keywords: Mercury; Amalgam; Removal; Dentistry; Face masks; Occupational health
Abbreviations: UAG: University Autonomous of Guadalajara; FO: Faculty of Dentistry; CLIO: Dental Clinic Odontology; CIDI: Research Center for Industrial Development
Introduction
Currently, there is a worldwide trend to rule out the use of mercury from human activities. In dentistry, there is a great controversy about the safety of the use of dental amalgam in patients and there have been attempts to demonstrate the occupational risk to which dentists are exposed by developing dentistry techniques that do not use mercury [1].
The fact that an amalgam contains mercury has raised some health and safety concerns. In assessing the occupational risk to which dental personnel are exposed to mercury, it has been observed that this depends on techniques used during the preparation of the amalgam and it has been determined that these risks can be minimized by performing modern practices complying with standards for Handling of hazardous substances [2].
Several cases of mercury poisoning and embryotoxicity among dental personnel have recently been documented [3]. In fact, exposure to inorganic mercury has been found to increase levels of this metal in blood plasma and urine [4]. In a dental practice, dentists and their dental assistants are at risk because of the use of mercury in the preparation of amalgam as they are chronically exposed to the vapor [5]. In Singapore, 96 dentists examined, exposed to 16.7 μg/m3 of mercury (Hg) in the air, were deficient in both memory and vision [6]. Dental workers, who work with amalgams, have a reduced fertility rate, low conceptionpossibilities, and their children have a lower IQ compared to thegeneral population [7]. A dental amalgam is an alloy of mercury,silver, copper, and tin and may also contain palladium, zinc, andother elements to improve handling characteristics and clinicalperformance. Amalgams have been used by dentists for over 100years because it is very durable and more accessible than otherdental restorative materials [2].
In a study by Svare et al. [8] there was an increase in mercurylevels in air exhaled by people with dental amalgams with aregistry of 0.01374 mg/m3 after chewing. That is, people withdental amalgams are potentially exposed to mercury with anaverage of about 13.74 μg/m3 every day [9]. At the dental clinic,concerns about toxicity problems include: inhalation of mercuryvapor and amalgam powder; ingestion of amalgam; allergy tomercury; and environmental considerations. Inhalation andingestion of mercury from dental amalgams may occur duringplacement, polishing, or removal of the restoration, or duringmastication [10].
According to OSHA (Occupational Safety and HealthAdministration), the permissible exposure limit is 0.1mg/m3 (vapor). NIOSH (The National Institute for OccupationalSafety and Health) stated that the maximum exposure limitor immediate danger to life or health limit is 10mg/m3 [8]. Inthe Rugg-Gunn study, the mercury vapor exposure level uponremoval of an amalgam restoration without water irrigation andwithout ventilation was 0.19 mg/m3, which is above the OSHAPermissible Exposure Limit [11].
Although the ADA has reported that amalgam restorationsused in dentistry do not necessarily pose a health risk, dentistsand dental staff should decrease mercury exposure to dentalamalgams [2].
The ADA also published an article on how to handle anddispose of amalgam waste [12]. These include (but are notlimited to): The use of traps for residual mercury, install amalgamseparators that comply with ISO 111432, use of a high suctionaspirator for the removal of amalgams, inspection and cleaningof the vapor traps and collect and recycle the dental amalgam.
The masks have the function of protecting our nasal mucosaand the entire oral cavity, avoiding the contamination by aerosolsoriginated by the rotary instruments of the dental office [13].
The facemasks, surgical mask, or hygiene mask are designedto avoid the spread of microorganisms that are lodged in themouth, nose, and throat avoiding the contamination betweenthe operator and the patient. They operate in dual forms, duringthe exhalation of the operator, the air of the nose and mouthexits at a certain speed and is directed frontally preventingits dissemination [14], and is capable of filtering particlessuspended in the air prone to aspiration by the operator as partof the contamination of the work area during dental treatment[15].
Facemasks have been used to reduce workers’ exposure toinfectious and toxic aerosol material occurring through splashesand droplets, primarily. However, their filtration capacity is verylimited because during the filtration tests they do not reach theminimum 95% required to provide respiratory protection [16],even when using up to five masks at the same time [17].
The particles that should be able to filter the masks shouldbe relatively thick, between 3 and 8 microns [14,15], and 0.1μmfor laser use (thus protecting the person using them againstsmoke of the laser) [18].
The facemasks are made of synthetic material and some havea polypropylene cover. Some can be smooth / pleated (othershave a rigid shape of the contour of the face) and are loopedaround the ears with elastic strings or with strips to better fit onthe nose and over the mouth. They are not designed to providea facial seal and thus allow filtration to enter through the edgesof the mask when the user inhales [18]. The objective of thispilot study is to identify the mercury levels in a dental operator’sfacemask at the end of removal an amalgam filling by detectionin an atomic absorption spectrophotometry at theOperatorysection of CLIO UAG during the month of July - September 2016.
Materials and Methods
Eight patients who required dental amalgam removaltreatment at the Operatory section of CLIO UAG were consideredin this pilot study. All patients received and signed informedconsent for the operative amalgam removal procedure. Theoperators were dental undergraduate students and postgraduateresidents of FO UAG who had indication of amalgam removal as anoperative treatment regardless of tooth. The personal protectionequipment for dental procedures was designated by the CLIOUAG, where the facemask was used as a specimen. Patients wereprotected with the use of a surgical disposable scrub gowns toreduce aerosol exposure during the dental amalgam removalprocedure.
Eight facemasks were obtained, made of polypropyleneof double cloth weighing 35 grams, with intermediate filter,pleated, with nasal adjuster, and an elastic cord to the ears. Thebrand name of the facemask is GRUPREYSA, and this was thefacemask used in the removal of dental amalgams. One new maskper patient was used as a control variable.
A rubber dam was used to isolate the tooth being treatedto reduce the amount of amalgam aspiration by the patientorally and respiratory. We used a high speed dental hand piecewith sterilized # 4 carbide ball for the removal of the amalgam.Constant irrigation and a conventional ejector were used for theaspiration of water during the procedure, with an average time of15 minutes per patient. The facemasks were removed accordingto the safety protocols of the CLIO UAG and immediatelydeposited in a self-sealed plastic bag to be taken for analysis.
An Atomic Absorption Spectrophotometer (EQQ 289) wasused for the identification of mercury ions in the facemasks at the Research Center for Industrial Development (CIDI) at UAG.The masks were weighed on an analytical balance individually.Subsequently the facemasks were placed in individual flaskand immersed in 50ml of 3% hydrochloric acid (HCl) for 24hours. Next the facemasks were moved and placed in to a 250mlvolumetric matrix containing: 0.5069g of sodium borohydride(NaBH4) and 2.5ml of dilute 5% sodium hydroxide (NaOH) was added. The contents were to be measured and dissolvedwith 250ml distilled water to determine the levels of mercurythrough the spectrophotometry study of atomic absorptionof the facemask used by the operators during the process ofdental amalgam removal. The data were analyzed using an excelspreadsheet to obtain central trend parameters.
Results
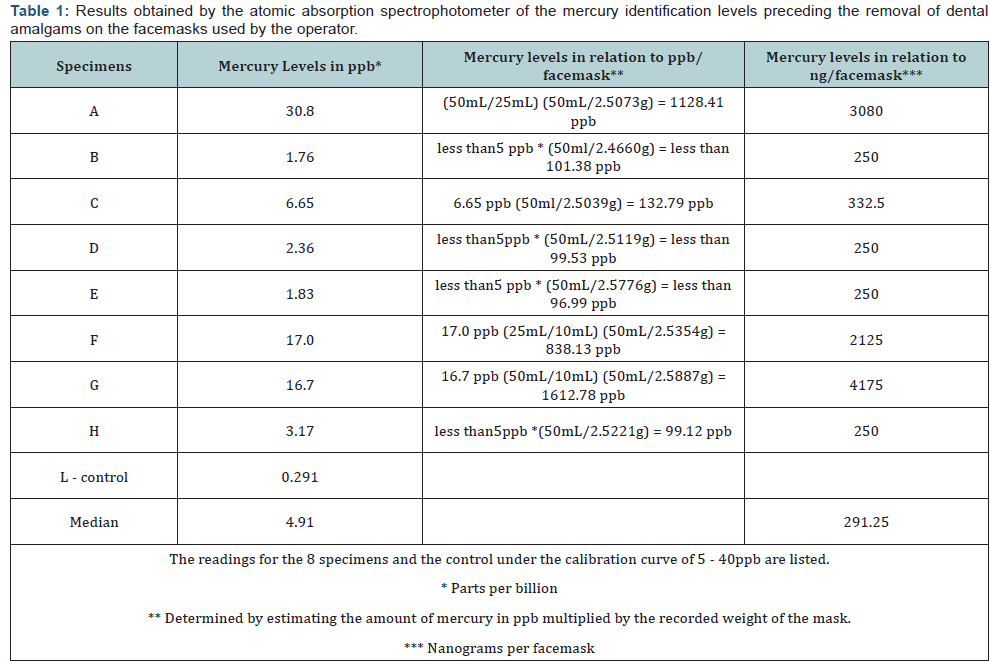
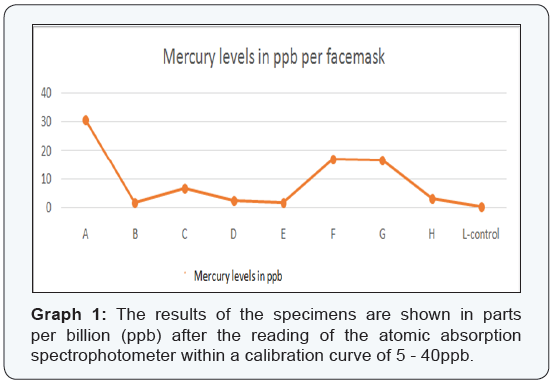
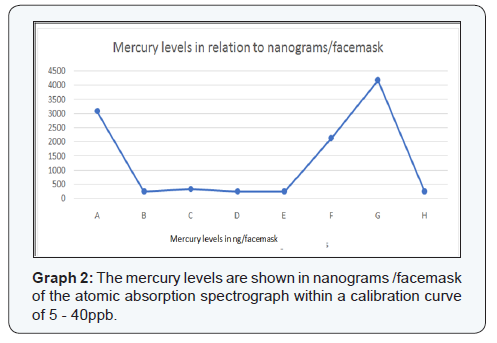
The results of this study are presented in Table 1. The 8samples were listed alphabetically, “A-H, and the control specimenwas labeled as sample “L.” The mercury levels identified by thespectrophotometer in the facemasks were shown as: parts perbillion (ppb), ppb in relation to the facemask, and in nanograms(ng) in relation to the facemask. Table 2 shows the class type(according to the Black classification of restoration) of eachtooth the amalgam was removed from by the operator for eachspecimen. The atomic absorption spectrophotometer performedtwo readings with a calibration curve of 5ppb - 40ppb. Thecontrol facemask had 0 ppb of mercury. Graph 1 shows specimensA, F, and G with the highest levels of mercury identified in thefacemasks (30.8ppb, 17.0ppb, and 16.7ppb respectively) with amedian of 4.91 ppb of all specimens. Graph 2 shows specimensB, D, E, and H with mercury levels of 250ng/facemask, with amedian of 291.25ng/facemask.
Discussion
Mercury is considered highly toxic to humans and candamage vital organ systems, including the nervous, digestive,respiratory, renal, and immune systems [19]. Thus, programs atthe international level are deliberately striving to eliminate theuse of mercury-added products.
Inhalation of elemental mercury vapor is the most relevantroute of mercury exposure. Mercury vapor in the breathing zone is inhaled into the lungs, where it is estimated that 80% isabsorbed by the lung tissues [20].
There is a significant correlation between mercurycontamination and work areas where mercury is produced athigh levels in environmental areas such as dental offices whereamalgam removal occurs [21]. Thus, inhalation of amalgampowder may contribute to mercury exposure in the workingenvironment. Ingestion and dermal exposure are also otherimportant routes of exposure to mercury.
In this study, the amount of mercury dispersed as aerosol inthe working environment was not considered, but the surfacemercury levels in the outer layer of the facemasks were analyzed.We deduced that the identification of the heavy metal on thesurface of the mask was the amount of mercury not inhaled bythe operator during the removal of amalgams.
It should be mentioned that the results of this study cannotbe compared with the results of Rugg-Gunn et al. The averageexposure level of mercury vapor when removing an amalgamrestoration, without water irrigation and without ventilation,is 0.19mg/m3 [11]. The results of this study show a median of291.25 ng/facemask and 4.91ppb in the reading of mercury ions.OSHA also expresses its results in vapor measurements in thework environment taken from the aerosols generated; however,the methodology of this pilot study used mass measurementthrough the atomic absorption spectrophotometer.
In this study, all samples were obtained using saliva ejectors.It is known that, using a saliva ejector during removal of amalgam,the operator is exposed to 168μm/m3. It also shows that usinga high-speed ejector reduces the amount of mercury to 1.5μm/m3 [20]. Thus, mercury vapor levels are considerably diminishedwith the use of a ejector or high speed air extractor [22].
The effectiveness of the face mask in terms of preventing themercury from reaching the airways was not evaluated in thisinvestigation. In addition, factors such as operator posture, useof suction systems, ventilation of the work area, contaminationof the facemask due to contaminated gloves, as well as theproximity between each dental unit were not considered asvariables for this study. However, in this pilot study, the type oftooth treated presents a subjective relation with the index ofmercury presented in the masks.
The three samples that had the highest mercury level werethose identified as A, F, and G; the three samples were obtainedfrom upper teeth (2 molars, class II restored with amalgam and1 premolar, class I restored with amalgam respectively) and theremoval time was different per the type and size of the amalgam.Sample “F” was collected from an operator who used a surgicalprotective mask during the removal of the amalgam, and yet itshowed high levels of mercury.
Sample “G” had the highest amount of mercury in terms ofng/facemask. We believe that this reading originated from the fact that the operator removed amalgams from three differentmaxillary teeth during the same session. The rest of the samplesonly involved the removal of one single amalgam tooth persession. Perhaps, removing more dental amalgams in a singlesession increases the levels of mercury identified on the surfaceof the masks, however, more samples would be needed to confirmthis claim.
We believe that the fact that no operator used indirectvision at the time of amalgam removal lead to a closer proximityof operator to patient to visualize the amalgam that had to beremoved. Therefore, this proximity resulted in a higher amountof contaminated aerosols reaching the operator’s face, leading toa higher mercury reading from the samples obtained from themaxilary teeth.
Surgical facemasks can help block larger droplets of particles,spills, sprays or splashes, which may contain germs, viruses andbacteria, so they won’t reach the nose or mouth. However, theyare used primarily to protect patients from the health workers,and reduce their exposure to saliva and respiratory secretionsand yet, these surgical facemasks do not create a tight sealagainst the skin or filter very small airborne pathogens, such asthose responsible for airborne diseases [23].
There are studies that show the relationship betweenthe manipulation and removal of amalgam per week with thelevels of mercury found in the dentist. Therefore, when anactivity poses a threat to human health or the environment,precautionary measures should be taken even if some cause andeffect relationships have not been established scientifically [24].
It is necessary for undergraduate programs in dentistry tofocus on courses, seminars or academic modules that addressissues of occupational safety, biosafety and infection control,as well as the risk of using hazardous substances such asmercury. Students should be capable of applying these protocolseffectively. This will benefit oral health professionals by reducingtheir exposure to this toxic heavy metal and will reduce theenvironmental impacts of mercury on solid waste, air, and waterresiduals.
Exposure to mercury and the possibility of reducing it in thedental workplace remains a challenge. This is a pilot study andthese results should be taken with caution and merit more casesand a methodological rigor for the control of the variables.
Conclusion
Levels of quantifiable mercury were identified on the outersurface facemask of the personal protective equipment whenremoving dental amalgams that round up to 291.25 ng/masks.
Greater number of samples and the establishment ofvarious variables (operator position, suction use) are needed tostatistically relate the impact of the mercury levels identified onthe facemasks after the amalgam removal.
Following the programs in occupational health, biosecurity and infection control in dentistry will allow us to work with highstandards of safety and quality in the work area.


Comments
Post a Comment