The Impact of Bisphosphonate Therapy Upon Oral Implant Treatment; A Systematic Review- Juniper Publishers
Juniper Publishers-Open Access Journal of Dentistry & Oral Health
The Impact of Bisphosphonate Therapy Upon Oral Implant Treatment; A Systematic Review
Sotirios N Kafantaris, Savvas N Kamalakidis, Nikolaos Ntabarakis, Argirios L Pissiotis and Nikolaos M Kafantaris*
Division of Prosthodontics Department of Removable Prosthodontics, Aristotle University Of Thessaloniki, School of Dentistry, Greece
Submission: December 01, 2016; Published: December 14, 2016
*Corresponding author: Nikolaos M Kafantaris, Division of Prosthodontics Department of Removable Prosthodontics, Aristotle University Of Thessaloniki, School of Dentistry, Greece, Tel: 302310832557; Email:nkafanta@dent.auth.gr
How to cite this article: Sotirios N K, Savvas N K, Nikolaos N, Argirios L P, Nikolaos M K. The Impact of Bisphosphonate Therapy Upon Oral Implant Treatment; A Systematic Review. Adv Dent & Oral Health. 2016; 3(2): 555610. DOI: 10.19080/ADOH.2016.03.555610
Abstract
Objectives: To conduct a systematic review in order to investigate the impact of systemic administration of bisphosphonates (BPs) upon the oral implant treatment.
Data: The MeSH terms used in combination were: dental implants and bisphosphonates, dental implants and osteonecrosis.
Sources: A literature search was conducted through the following data bases: Cochrane Library, Pubmed/ Medline, Biomedical data base Elsevier from 1966 to November 2015. The literature search was completed by hand search.
Study selection: Inclusion criteria: In vivo human studies were selected with titles including the terms oral/dental implant in conjunction with bisphosphonates/diphosphonates, alendronate, etidronate, zelodronate, clodronate, risedronate, ibandronate, pamidronate and the terms oral/dental implant in conjunction with osteonecrosis of the jaw. The literature search rendered a total number of 470 articles. From those articles, only 29 of moderate to weak strength of evidence were included in the study.
Conclusions: Based on the current data, the following conclusions can be drawn: The longer duration of the drugs’ use and the IV administration could be considered as negative factors. The placement of dental implants in posterior jaw regions could also be considered as negative factor, for the success of the implant treatment. In cases with bisphosphonate-related osteonecrosis of the jaw it appears that the cause could be attributed to the functional loading of the dental implant and not the surgical procedure. . The risk of developing BRONJ is greater when the implant placement is performed during BP therapy
The history of BPs use orally, cannot be considered as an absolute contraindication to implant placement therapy, since moderate to weak strength of evidence support this notion.
The history of BPs use intravenously according to several guidelines [1] is an absolute contraindication to implant placement therapy ,However only moderate to weak strength of evidence support this guidelines.
Keywords: Bisphosphonates, Dental implant, Success rate, Survival, Failure, Osteonecrosis of the jaw
Clinical Significance The impact of bisphosphonates intake upon the survival and success of dental implants and the associated risk of bisphosponate- related osteonecrosis of the jaw development should be carefully assessed by the clinician at the treatment planning and decision making stage.
Introduction
TBisphosphonate (BP) prescriptions in the US are estimated at circa 30 million per year [1]and at more than 190 million worldwide [2]. The majority of patients under BP treatment are over the age of forty and therefore potential candidates for implant supported dental prostheses due to tooth loss. These facts point to an increased interest of the dental community, as well as the public, to the possible interaction that may exist between dental implant treatment and BP medications.
The specific question that mostly concern dental practitioners is the following: To what degree osseointergration of dental implants is affected by BP medication in relation to primary stability and long term success rate? The frequency of bisphosphonate-related osteonecrosis of the jaw (BRONJ) is reported, for intravenous administration of BPs, to range between 1-15% [3] while for the oral BPs the figure is extremely low (1/10000 pts) [2]. Most incidents of BRONJ are observed after tooth extraction (57%) [4]. Predisposing conditions, including rheumatoid arthritis, diabetes mellitus, glucocorticoid therapies, and disease-modifying antirheumatic medications, co-exist in more than half of the cases [4]. Since the surgical procedure for dental implant placement can be considered more traumatic compared to tooth extraction, the question exists if these patients are in danger of developing BRONJ and to what extent. Furthermore, to what extend the negative confounding factors (i.e. old age, diabetes, use of corticosteroids, smoking, the duration and potency of BP treatment, and oral hygiene status), may affect the occurrence of BRONJ to those specific patients [3,5,6].
According to the guidelines of the Cochrane Library Tutorial, the first crucial step for a successful systematic review is the wording of the correct clinical question (PICO) [7] The PICO question should always be expressed in the following format:
- P. Patient/population,
- I. Intervention/indicate,
- C. Comparator/control,
- O. Outcome.
Up to date, four systematic reviews related to the topic of BP administration in conjunction to dental implant treatment have been published by: Madrid & Sanz [8] Javed & Almas [9] Chadha et al. [10] & Ata- Ali et al. [11]. These systematic reviews solely investigated: a) the success rates of implant placement in patients with a history of BP medication and b) the incidence rate of BRONJ to patients treated with dental implants.
In the review by Javed & Almas [9] the PICO question included the degree of the osseointergration and long term stability of dental implants in patients treated with BPs. Similarly in the review by Ata- Ali et al. [11] the question investigated was: “what is the impact of BP therapy upon dental implant survival”. Madrid & Sanz [8] also investigated a single PICO question divided into two parts. The first part targeted the successful osseointergration of dental implants and the second part investigated whether the placement of dental implants can initiate the development of BRONJ. In the review by Chadha et al. [10] the two parts of the PICO question, set in the review by Madrid and Sanz8, were formulated into two separate PICO questions.
Since further studies related to the clinical aspects of the topic may have been published, subsequent to the aforementioned systematic reviews, it was deemed necessary to conduct an up to date systematic review to include the most recently published studies.
Materials and Methods
Objectives
The purpose of the current systematic review was to test the null hypothesis of no difference in the implant failure rates, the BPs’ administration route and the temporal association between the occurrence of BRONJ and the point of dental implant insertion, for patients receiving or not receiving BPs, against the alternative hypothesis of a difference. The following PICO questions were created.
In the selected topic of the current systematic review the formulation of the clinically significant questions into PICO questions created the following PICO questions:
PICO question 1: Are patients with a history of BP medication use, (orally or intravenously), equally appropriate candidates for successful osseointergration and long term survival of dental implants compared to patients not being treated with BPs?
PICO question 2: What is the risk of developing BRONJ and how is that risk differentiated in relation to the drugs’ administration route (oral vs. intravenous) in patients with previous history of BPs use, who receive dental implants?
PICO question 3: In patients which had dental implants placed before the initiation of BPs medication, do those implants have the same long term survival rates when compared with implants placed in patients with no history of BPs use?
PICO question 4: In patients who developed BRONJ, because of BPs medication, is dental implant placement applicable?
The methodology carried out in the present study included:
- Literature search
- Application of inclusion and exclusion criteria
- Quality assessment
- Evaluation of search results
Search Strategies
The literature search was conducted through the following data bases: a. Cochrane Library, b. PubMed/Medline and c. Embase Biomedical data base/Elsevier. The search was conducted up to November 30 2015. The MeSH terms used were: dental implants and bisphosphonates, dental implants and osteonecrosis. It should be noted that the BPs alendronate, etidronate, zelodronate, clorodronate, ibandronate, risedronate, pamidronate were included under the MeSH (Medical Subject Heading) Bisphosphonates. No restrictions were placed on language or date of publication.
Inclusion and Exclusion Criteria
Articles were selected if the title included: the terms oral/dental implant in conjunction with bisphosphonates/ diphosphonates, alendronate, etidronate, zelodronate, clodronate, risedronate, ibandronate, pamidronate or the terms oral/dental implant in conjunction with osteonecrosis of the jaw In vitro or animal studies, narrative reports or literature reviews. Articles with cases of osteonecrosis related to other treatments than dental implants were also excluded.
Study Selection
Two examiners (SNK1 and SNK2) independently read the titles and abstracts of all articles. The literature search was completed by hand accessing the references cited in all identified publications fulfilling the inclusion criteria. Any disagreement between the reviewing authors was resolved by consensus among all authors. For the studies that met all the inclusion criteria the full report was reviewed.
Quality Assessment
Quality assessment of the articles was executed according to the Oxford Centre for Evidence-Based Medicine-Levels of Evidence [12]. The OCEBM was used to calculate the study quality utilizing the following criteria: prevalence, diagnosis, prognosis, treatment and screening. It categorized the articles into five levels (1-5) based on the aforementioned criteria.
Results
The initial search revealed a total number of 470 original articles. From those articles and based on their title, 422 articles were excluded as not being relevant to the topic. From the remaining 48 articles and based on their abstracts, 20 articles were also excluded. A quality assessment was performed to the remaining 28 articles and to one additional article found by hand searches totaling to 29 articles included in the study (Table 1).
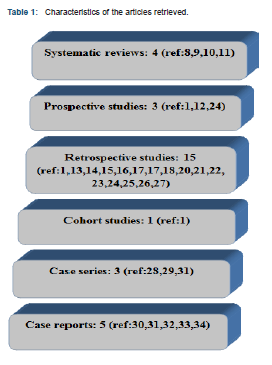
Because of the limited quantity of the retrieved investigations and the heterogeneity of those studies, a meta-analysis could not be made and the data will be presented in a descriptive manner. The characteristics and classification of the articles based on the separate PICO questions are presented in Table 2.
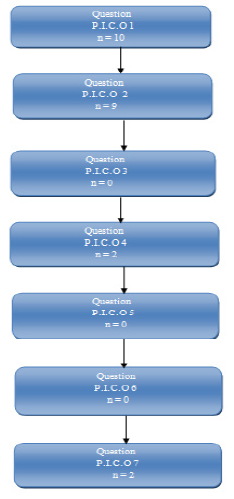
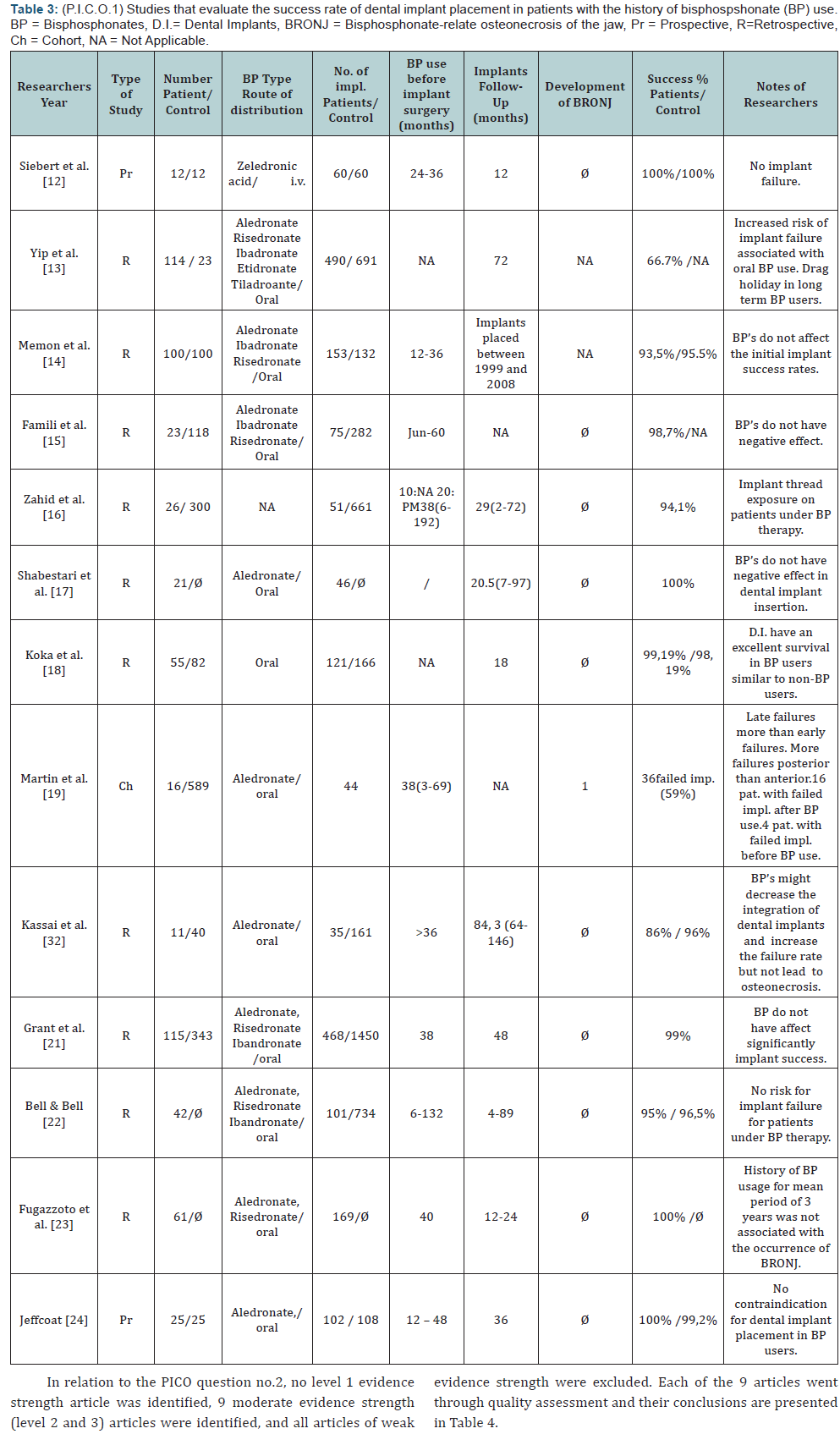
In relation to PICO question no.1, no level 1 evidence strength article was identified, 13 moderate evidence strength (level 2 and 3) articles were identified, and all articles of weak evidence strength (case reports) were excluded. Each of the 13 articles went through quality assessment and their conclusions reached by consensus are presented in Table 3.
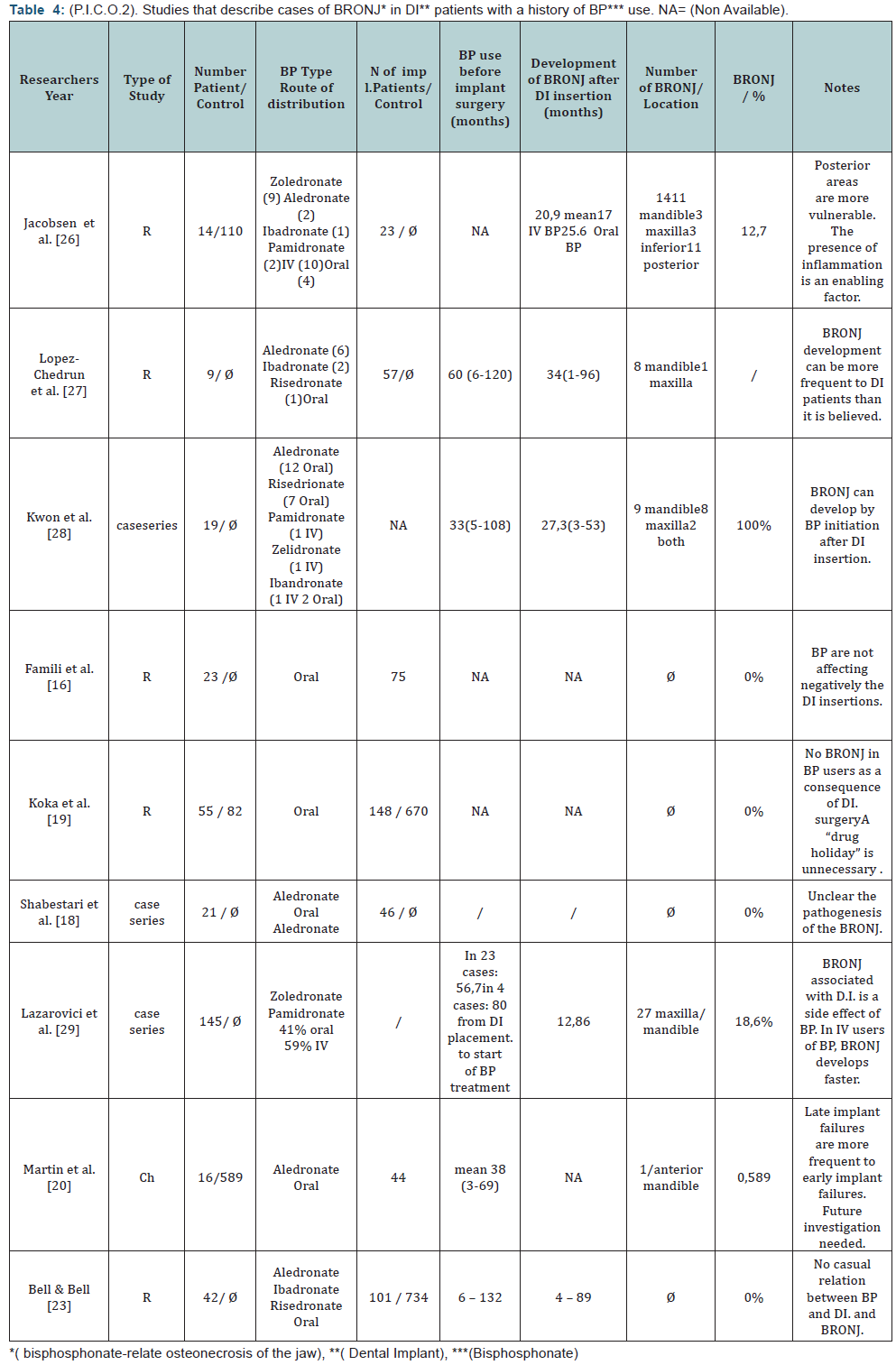
In relation to the PICO question no.2, no level 1 evidence strength article was identified, 9 moderate evidence strength (level 2 and 3) articles were identified, and all articles of weak evidence strength were excluded. Each of the 9 articles went through quality assessment and their conclusions are presented in Table 4.
In relation to PICO question no.3, no level 1-3 evidence strength articles were identified. In a case series article by Shabestari et al. [13] in 14 patients, implants were placed before BP treatment and they were followed up for 4.2 years (range 0.6-8.1). All implants were stable and free of symptoms. They concluded that “time of BP therapy before and after implant placemant showed no statistically significant influence on implant treatment”. Kwon et al. [14] in their case series study identified 3 out of 19 cases in which BRONJ developed after BP initiation following implant placement. In another case series article, by Goss et al. [15], in which the authors collected data through a questionnaire sent by mail, it was calculated that 0.89% of the patients had an implant failure, based on the assumption that 5% of the patients were taking bisphosphonates orally. The true value was 1 in 2,286 patients (0.04%). Specifically, they identified 7 cases of oral bisphosphonates-associated implant failure. Worth mentioning was that in 4 individuals the dental implants were successfully osseointergrated before the start of the BP medication. That constituted an indirect report to the negative effect of the BPs medication to the survival of fully osseointergrated dental implants.
In a retrospective case series by Holzinger at al. [16] was also identified, where they treated surgically 138 patients for BP associated osteonecrosis of the jaw, between April 2004 and July 2012.Among them thirteen patient had dental implants(total 47 implants). The implants were inserted before( 3 cases, 8 implants)group 1,during (2 cases, 19 implants) group 2, or after Bp therapy(7 cases, 20 implants)group 3. In 12 patients BRONJ developed in the mandible and in 1 patient in the maxilla .No spesiphic information was given about the location of the implant placement. All patients were woman and all patients in group 3 were smokers.
The 3 cases of initiation of BP therapy and BRONJ development after implant placement were all cases of iv BP therapy They concluded that the insertion of dental implants during or after BP treatment accelerate the development of BRONJ. BRONJ occurs less frequently when the implants had been inserted before the initiation of BP therapy.
Finally, a case report by Subramanian et al. [17] was also identified, where all of six dental implants which were placed in the mandibular anterior region failed simultaneously. The implants were initially placed in 2003 and a fixed detachable dental prosthesis was fabricated. The patient visited her general dentist annually for her recall appointments with no signs of peri-implantitis or occlusal prematurities. In 2011 the patient presented to the prosthodontist, complaining of loosening of her prosthesis and a moderate to high jaw pain. Clinical exam showed no signs of inflammation or BRONJ symptoms. A panoramic radiograph confirmed extensive osteolysis around all 6 implants, which were easily removed. The authors indicated that the patient started osteoporosis treatment in 2004 with Fosamax (alendronate 50mg/wk and in 2009 increased to 70mg/wk), which continued till the loosening of the implants. This paper, although a case report, directly linked the failure of dental implants with the beginning of BPs treatment at a later stage of the implant placement.
In relation to the PICO question no.4, three case reports were identified. Rugani et al. [18] reported a case of successful implant treatment in the posterior maxilla after the complete healing of the adjacent area treated for BRONJ. The patient had been treated for osteoporosis with 3mg ibandronate intravenously every 3 months for a 20 month period. BP treatment was terminated after the onset of BRONJ. Ferrari et al. [19] used dental implants to successfully reconstruct a total mandibulectomy site, as a result of BRONJ from intravenous BP medication complicated by a mandibular fracture. Marx [20] used dental implants and grafting materials for the rehabilitation of a case with a mandibulectomy site due to BRONJ. The author proposed the use of implants under the condition that the BPs will be replaced by alternative osteoporotic medication.
Discussion
The present data of the BPs’ effect on dental implant rehabilitations comprises of moderate to weak level evidence studies (retrospective studies, case series and case reports). It remains to be determined whether the use of BPs could be a deterrent factor to the placement of dental implants, especially if that affects equally patients that use them orally or intravenously.
In relation to whether BPs affected the course of implant treatment (PICO question 1), no certain, conclusion could be drawn, based on the published reports. All papers included in this study, clearly pointed that short term success rates were positive and comparable to those in patients without BP treatment. Many of the authors [13,21-27] suggested that implant placement in patients under BP medication could be considered “safe and predictable” and that a drug holiday was not necessary. It must be pointed out though that the sample size was relatively small, the follow up period short and the evidence level of those papers moderate to weak (level 3 or less).
In relation to PICO question 2, what is the danger of BRONJ incidence in patients already undergoing BP treatment and dental implants are placed, and how is that differentiated between oral and intravenous administration, again it must be pointed out that no safe conclusion can be drawn, because of the lack of strong level evidence studies (level 1 or 2). From the studies included, Lazarovici et al. [28] presented a series of 27 clinical cases with BRONJ in patients with dental implants under BPs medication. In 77.8% of those cases BRONJ developed after at least a six month or longer period from the time of implant placement. Patients with IV administration of BPs developed BRONJ earlier than those with oral administration. In 4 out of 27 patients, implant placement was initiated on average 80 months prior to the beginning of BPs use. The authors suggested that for those patients, the cause of developing BRONJ was the functional loading of the dental implants and not the surgical implant placement procedure, which differs from that of tooth extraction. Similar findings can be concluded from the study by Jacobsen et al. [29]. The mean time period from the implant placement to the development of BRONJ was 21 months. That period was shorter (17 months) for patients with IV use of BPs, compared to those with oral use (26 months). In the same study 9 out of 12 patients had implant failure in the posterior region. Similarly, in the study by Lazarovici et al. [28] 19 out of 27 patients with multiple implants experienced failure in the posterior jaw region. Similar results were presented in the study by Martin et al. [30] in which from the 44 placed implants the distribution for the 26 failed implants was 18 for the posterior region (9 maxilla, 9 mandible) and 8 for the anterior region (3 maxilla, 5 mandible). The findings of the study by Shabestari et al. [13] suggested that the site of implant placement (posterior jaw region) although not statistically significant presented a risk factor for the development of BRONJ. In this study in 21 females with osteoporosis and under oral BPs medication, 46 dental implants were placed with no incident of BRONJ. 32 out of the 46 implants were placed in the anterior region.
Jacobsen et al. [29] stated that the microbial factor was the main cause of bone pathology, since it could be difficult to perform a thorough personal hygiene regimen to the implants in the posterior regions, which may lead to peri-implantitis and subsequently to “osteopathology” of the jaw bone. The 12 cases,that were examined histopathologically, in the Jacobsen study [29] exhibited acute or chronic inflammation, with evident presence of Actinomyces. The fact that the risk factor of developing BRONJ was more significant in the posterior jaw region [28,30] in relation to the fact that BRONJ could develop many months after the implant surgeryled Jacobsen et al. [28] to this conclusion [28]. Therefore, they considered that it cannot be classified as osteonecrosis, of the jaw, but simply as osteopathology. That statement could be at odds with the opinion expressed by Marx [20] who believed that BRONJ was the result of insufficient self defense of the bone tissue locally, that cannot cope with the increased metabolic demands caused by various factors (trauma, mechanical loading, inflammation) He termed this clinical condition as BRONJ “Bisphosphonate related osteonecrosis of the jaws”. Marx’s opinion was based on the fact that BRONJ was often observed in patients under BPs medication with tori palatini or tori mandibularis, and also under the pressure areas of the bases of removable prostheses, where the microbial factor was considered absent [19]. The fact that most cases of BRONJ developed in the posterior jaw areas, point out that beside the microbial factor and /or trauma, the mechanical overloading of the implants, might also be a factor for BRONJ development. Based on the current published data, which consisted of moderate to weak level evidence studies (retrospective studies, case series ,case reports) no safe conclusions, as whether the use of BP medication should be a contraindication for dental implants placement could be drawn.
The same applies to whether the surgical placement could cause the development of BRONJ.
Finally, during the formulation phase of the PICO questions, it was noted that some of the questions produced no papers, thus were not included in the review. Those questions were as follows [31-34]:
- In patients with a history of orally administrated BPs, what are the chances of successful osseointergration and long term survival of dental implants placed, in comparison with implants placed in patients with a history of IV administrated BPs?,
- In patients with long term recorded use of BPs, when dental implants are placed, do they have the same success and long term survival rates, when compared with dental implants placed in patients who recently initiated treatment with BPs?, and
- In patients who are currently under BPs medication and stop for a short period (drug holiday), do they have better success and long term survival rates of their dental implants, compared with patients under constant and long term use of BPs? That finding was particularly important for highlighting gaps in the current literature and should be assessed in future clinical studies.
Conclusions
Through the literature search it became apparent that for safe answers to be given to the clinical questions that the present review dealt with, clinical studies of higher evidence level are needed (levels 1 and 2).
Based on the current available data the following conclusions could be drawn with caution (reservation):
- The longer duration of the drugs’ use and the IV administration could be considered as negative factors.
- The placement of dental implants in the posterior jaw regions could also be considered as a negative factor for the success of implant treatment.
- In case of developing BRONJ it seemed that the primary cause was the dental implant itself (functional load) and not the surgical procedure.
- The history of BPs use orally, cannot be considered as an absolute contraindication to implant placement therapy, since moderate to weak strength of evidence support this notion.
- According to several guidelines the history of BPs use intravenously is an absolute contraindication to implant placement therapy, However only moderate to weak strength of evidence support this guidelines.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Open Access Journal of Dentistry & Oral Health please click on:


Comments
Post a Comment