Fluocinoloneacetonide 0.01% and Dexamethasone 0.1% Mouthwash in the Treatment of Symptomatic Oral Lichen Planus- JuniperPublishers
Juniper Publishers-Open Access Journal of Dentistry & Oral Health
Abstract
Purpose:: The objective of this study was to compare the effectiveness of fluocinoloneacetonide0.01% and dexamethasone 0.1% mouthwash in treating symptomatic oral lichen planus (OLP).
Patients and Methods: Thirty-four patients (27 females and 7 males; mean age 47.26±11.78 years) with symptomatic OLP were treated for 6 weeks with either fluocinoloneacetonide0.01% mouthwash or dexamethasone 0.1% mouthwash in a randomized, double-blind, clinical trial. Pain severity and lesion size and severity were assessed using the VAS pain score and clinical score, respectively.
Results: At the end of the treatment period, pain symptoms (VAS pain score) and lesion size and severity (clinical score) were significantly lower in the fluocinoloneacetonide 0.01% and dexamethasone 0.1% mouthwash groups compared with baseline. However, the difference in these scores between the groups was not significant.
Conclusion: fluocinoloneacetonide 0.01% and dexamethasone 0.1% mouthwash were effective in treating symptomatic OLP. However, additional studies using a larger population and a longer treatment period and follow-up are needed to confirm these findings.
Keywords: Dexamethasone; Fluocinoloneacetonide; Mouthwash; Oral lichen planus
Introduction
Lichen planus is a chronic inflammatory disorder of the cutaneous and mucosal tissues. Oral lichen planus (OLP) is an immune mediated disease of unclear etiology, in which epithelial cells are recognized as foreign due to changes in their cell surface antigenicity [1]. The prevalence of OLP varies from 0.3%–2.3% of the general adult population and has a higher prevalence in middle-aged females [2]. Oral mucosal OLP lesions commonly occur bilaterally on the buccal mucosa, and frequently appear on the tongue, gingiva, mucobuccal fold, or at multiple sites. The clinical appearance of OLP has 5 different forms: 1) reticular (that is usually asymptomatic), 2) papular, 3) plaque, 4) atrophic / erythematous and 5) erosive / ulcerative that typically has persistent symptoms [3].
In contrast to cutaneous lichen planus, whose clinical course is often mild and resolves within 2 years, mucosal OLP tends to follow a more chronic course and is often has acute lesions characterized by pain and a burning sensation resulting in a considerable loss of quality of life for the patient [4]. The diagnosis of OLP is based on a combination of characteristic clinical findings and histopathological examination.
Because OLP is a chronic disease, various treatment regimens have been used, however, a complete cure is difficult to achieve [5]. Because of the anti-inflammatory and immunosuppressive effects of corticosteroids, topical corticosteroids are considered as the first choice for the management of symptomatic OLP to relieve inflammation, discomfort, and pain, as well as to reduce the side effects of systemic corticosteroids [6]. Previous studies have shown that the use of fluocinoloneacetonide in orabase (FAO) in treating OLP resulted in clinical improvement without serious sideeffects [7,8]. Thongprasom et al. found complete remission in 77.3% of OLP patients after a 2-year treatment using FAO 0.1% [7]. Furthermore, rinsing with dexamethasone 0.1% mouthwash for 6 weeks has been reported to significantly reduce the levels of cytokines, including TNF-α, IL-1α, IL-6, and IL-8, in the saliva of OLP patients [9].
The objective of this randomized, double-blind, 6-week clinical trial was to compare the treatment efficacy of fluocinoloneacetonide 0.01% and dexamethasone 0.1% mouthwash on symptomatic OLP.
Patients and Methods
Patients
Thirty-four OLP patients were enrolled in this study. All patients signed informed consent forms prior to beginning the study. The study protocol was approved by the Ethics Committee of the Faculty of Dentistry, Chulalongkorn University. The OLP patients consisted of 27 women and 7 men with a mean age of 47.26 ± 11.78 years, ranging from 19–73 years. The mean duration of the disease was 12.74 ± 24.61 months, ranging from 0.06 to 120 months.All samples were clinically diagnosed and histopathologically confirmed as erosive/ulcerative OLP lesions without dysplasia. None of the patients had systemic diseases, took any medications, or received prior treatment for OLP.
Inclusion and exclusion criteria
Patients were enrolled in the study if theypresented with symptoms of histopathologicallydiagnosed oral lichen planus [1] and were willing to participate in the study.
A patient was excluded if they had histopathologic signs of dysplasia, systemic diseases, took any medications, or had received prior OLP treatment.
Interventions and assessments
The individuals who met the eligibility criteria were randomly divided into two groups. Randomization was done by simple randomization where random numbers were generated from a random table and the patients were allocated using the sealed envelope method. Sixteen patients were instructed to rinse with fluocinoloneacetonide 0.01% mouthwash (FA) and the remaining 18patients were instructed to use dexamethasone 0.1% mouthwash (DX). The patients were instructed to rinse with 5 ml of their respective mouthwash for 1 min and then expectorate, three times a day over a period of 6 weeks, and instructed not to eat or drink for 30 minutes after each application.
The patient’s most severe and extensive lesion was identified as a marker lesion, and assessed for areas of reticulation, erythematous, and ulceration by visual examination using clinical scoring [7] as follows:
- Score 5: white striae with an erosive area >1 cm2.
- Score 4: white striae with an erosive area <1 cm2.
- Score 3: white striae with an erythematous area >1 cm2.
- Score 2: white striae with an erythematous area <1 cm2.
- Score 1: mild white striae only.
- Score 0: no lesions, normal mucosa
Evaluations were performed at three time points:
- before treatment (baseline)
- 4 weeks after beginning treatment
- 6 weeks after beginning treatment
At each follow-up visit, the patients were asked about any unwanted side effects, such as a burning sensation or alteration in their sense of taste, their marker lesions were assessed by clinical scoring, and their subjective symptoms were evaluated using a visual analogue scale (VAS). The patients indicated the severity of their pain and/or discomfort on a 100 mm VAS. The scale, a 100 mm horizontal line, was designed with zero discomfort (no pain) on the far left of the horizontal line and 10 (worst pain imaginable) on the far right. The patients made a vertical line across the point that they felt represented their symptoms. All clinical evaluations were performed by the same investigator, who was blinded to the patient’s assigned treatment.
Criteria for the assessment of the remission of OLP lesion
The OLP lesions were clinically assessed after the treatment according to the criteria set by Thongprasom et al. complete remission (CR)-no symptoms or very mild symptoms, lesions resolved or only mild white striae present; partial remission (PR)-symptoms decreased, mild white striae and erythematous areas present; no response (NR)-symptoms persisted with no improvement or the lesions were worse [7].
Statistical analysis
Statistical analysis was performed using the SPSS 22.0 software package (SPSS Inc., Chicago, IL, USA). The severity scores for pain, burning sensation, and mucosal extension were ordinal, thus, they were analyzed using nonparametric tests. The proportions of symptom scores for each treatment group were analyzed at each time interval (baseline, 4 weeks, and 6 weeks) using the Wilcoxon signed-rank test. The Mann–Whitney U-test was used to statistically compare symptom scores between treatment groups at each time interval. A p value of less than 0.05 was considered significant. The primary end-point was the number of patients who achieved complete remission (severity score of 0) of symptoms/signs.
Results
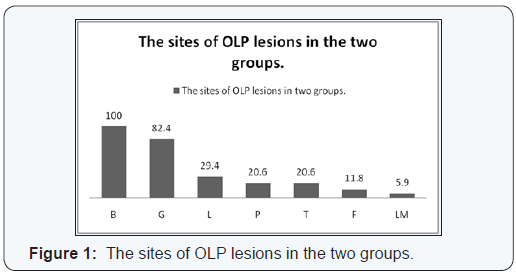
B = buccal mucosa, G = gingiva, L = lips, P = palate, T = tongue, F = floor of the mouth and LM = labial mucosa
OLP lesions were most commonly found on the buccal mucosa (100%), gingiva (82.4%), lips (29.4%), palate (20.6%), tongue (20.6%), labial mucosa (5.9%), and floor of the mouth (11.8%) (Figure1). Most patients had more than one lesion site.
The VAS pain and burning sensation scores of the patients in the treatment groups are illustrated in Figure 2. There was no significance difference in the pain and burning sensation scores between the two groups (p> 0.01) at baseline. A significant difference in the VAS pain score was found between baseline and week 6 in both treatment groups (p< 0.01). However, no significant difference in the VAS pain score was found between the two groups (p> 0.01).
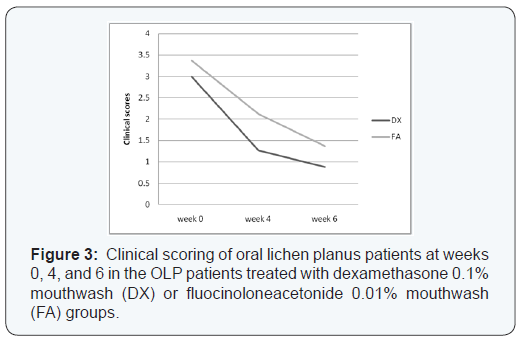
Table 1 shows the results of the clinical scores of the treatment groups. Considering each treatment separately, there was marked improvement in all efficacy outcome measures throughout the duration of the study. Although the DX group tended to have lower scores compared with the FA group, there was no significant difference in the mean scores between the two groups at baseline or week 6 (p>0.05). However, the difference in the mean scores between baseline and week 6 was significant in both groups (p< 0.05) (Figure 3).
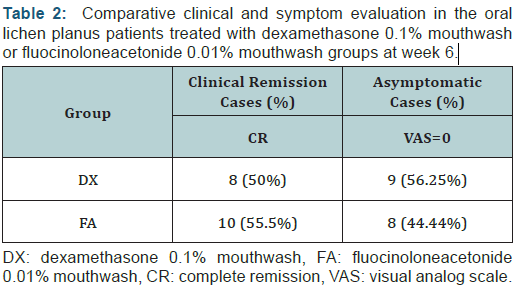
When the results of each efficacy outcome measure at the end of treatment were compared with the baseline data within each treatment group, they showed significant improvement in all outcomes at 4 and 6 weeks (p< 0.05). At the end of 6 weeks, complete remission was seen in 8/16 (50.0%) patients in the DX group compared with 10/18 (55.5%) patients in the FA group (Table 2). At the end of 6 weeks, an asymptomatic state (VAS = 0) was achieved in 8/18 (44.44%) patients in the FA group and 9/16 (56.25%) in the DX group. There was no significant difference in disease remission (p > 0.05) between the two topical steroids. No clinically detectable atrophy, telangiectasia, taste sense alterations, or allergic reactions were found in the treatment groups. In the DX treatment group, three (18.75%) patients developed secondary candidiasis that was successfully treated with topical antifungal agents. In contrast, no patients in the FA group developed secondary candidiasis.
Discussion
Lichen planus is a chronic inflammatory mucocutaneous disease. Mucosal LP lesions are occasionally complicated by extensive, painful erosions, which cause a considerable decrease in the quality of life of the patient. The oral mucosa, genital mucosa, conjunctiva, esophagus, larynx, and anus may be affected by LP. In contrast to cutaneous lesions, erosive mucosal lichen planus lesionsare highly resistant to topical treatment, tend to have a chronic course,and rarely resolve spontaneously. In addition, erosive mucosal lichen planus lesions may have a slightly increased risk of malignant transformation [3,10]. The clinical and epidemiological characteristics seen in the present study (patient age, sex, symptoms, lesion location, and characteristics) are in line with the findings of previous studies [7,8]. The patients in our study commonly complained of a burning sensation and pain before treatment was initiated.
The aim of OLP therapy is to eliminate atrophic and ulcerative lesions, alleviate symptoms, and potentially decrease the risk of malignant transformation. Although various OLP treatments have been used, these have not always generated good results. OLP treatments include topical retinoid therapy, which is less effective compared with topical corticosteroids therapy. In additional, retinoids may have adverse effects when administered systemically [11,12]. Low-dose cyclosporine mouth rinses have been reported to be effective; however, these are not widely used because of their high cost and immunosuppressive effects, and are not more effective compared with topical corticosteroids [13,14]. Despite these various alternative treatment options, the treatment of choice for OLP remains corticosteroid therapy [2,15,16]. Topical corticosteroids effectively penetrate the squamous epithelium, and have less severe side effects than those of systemic corticosteroids due to their low systemic absorption. There have been numerous studies on the efficacy of various corticosteroids including triamcinolone acetonide, fluocinoloneacetonide, fluocinonide, clobetasol propionate, mometasonefuroate, and hydrocortisone hemisuccinate as mouthrinses, ointments, gels, and orabase in treating erosiveulcerative OLP [2,15,16].
Ora-/ointment-based forms are difficult to use, especially for multiple site OLP, and many patients are uncomfortable with the sticky sensation of these formulations. Therefore, other forms of topical corticosteroids have been investigated, such as aqueous suspensionsand alcohol-based mouth rinses. Many studies showed similar clinical efficacy compared to topical treatment, but better acceptability, when using a corticosteroid mouthwash. Rinsing with dexamethasone 0.1% mouthwash for 6 weeks was shown to significantly reduce the levels of cytokines, including TNF-α, IL-1α, IL-6, and IL-8, in the saliva of OLP patients [9].
In the present study, dexamethasone 0.1% mouthwash andfluocinoloneacetonide 0.01% mouthwash both resulted in a reduction in painful symptoms (VAS pain score) and lesion size and severity (clinical score) during the 6-week period. The difference in these scores between groups was not significant at the end of the treatment period. However, the fluocinoloneacetonide 0.01% and dexamethasone 0.1% groups both showed better results compared with baseline. The findings from our study suggest that fluocinoloneacetonide 0.01% mouthwash and dexamethasone 0.1% mouthwash can be used as a first choice to manage symptomatic OLP.
Importantly, successful OLP management depend upon patient cooperation. Oral hygiene control during topical corticosteroid administration, and advising these patients to avoid precipitating factors such as spicyfoodandhaving broken restorations replaced were found to be of benefit in treating OLP Several studies have considered the immunosuppressive effects of topical corticoid treatment, which in some cases led to an alteration in the oral flora from excessive growth of fungal species, mainly C. albicans, resulting in candidiasis [10,17]. Therefore, some reports have proposed the parallel administration of antifungal agents during corticosteroid treatment [2,18]. The incidence of candidiasis in the present study was 18.75% (3/16), which was similar to or lower than those of previous studies using topical corticoids [6-8,19].
Conclusion
Dexamethasone 0.1% and fluocinolone acetonide 0.01% mouthwash was effective treatment for oral lichen planus. In addition, the incidence of fungal over-infection was low, and was eliminated by topical antifungal agents. However, additional larger studies, with a longer treatment period and follow-up, are needed to confirm these findings.
Acknowledgements
The authors are grateful to Professor Kobkan Thongprasom for her helpful advice. The authors thank Dr. Kevin Tompkins for critical review of this manuscript.
For more Open Access Journals in Juniper Publishers please click on: https://www.zoominfo.com/c/juniper-publishers-inc/370428819
For more articles in Open Access Journal of Dentistry & Oral Health please click on:


Comments
Post a Comment