Nanoparticles and their Applications in Orthodontics- Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DENTISTRY & ORAL
HEALTH
Nanoparticles and their
Applications in Orthodontics
Authored by Panchali Batra
Abstract
Nanoparticles (NPs) are insoluble particles
smaller than 100 nm in size and the set of technologies that enables
manipulation of these particles on an atomic, molecular and supra
molecular scale is termed as ‘Nanotechnology’. Applications of
nanotechnology are being ventured in various domains including health
care and have also carved their way into various specialties of
dentistry. This article presents an insight into various types of
nanoparticles and their application in the field of Orthodontics. The
various tests performed when using nanoparticles, to detect the physical
and biological properties of the new material, are also summarized for
easy referral.
Keywords: Nanoparticles; Nanotechnology; OrthodonticsAbbreviations: NPs: Nano Particles; MRI: Magnetic Resonance Imaging; USP: Ultrasonic Spray Pyrolysis; NFA: Nano Sized Fluoroapatite; NFHA: Nano Fluorohydroxyapatite; PSPMA: Polymer 3-Sulfopropyl Methacrylate Potassium Salt; RMGI: Resin Modified Glass Ionomer Cement; FN: Fluoride Releasing Nanofilled Composite; AFM: Atomic Force Microscope; Bio MEMS: Biomedical Micro Electro Mechanical Systems; NEMS: Nano Electro Mechanical Systems
Scope of this Review
This article presents a brief overview on basic
definitions related to field of nanotechnology, types of nanoparticles,
nanotechnology and types of nanoparticles. The focus of this article is
on application of nanoparticles in orthodontics. Though there are many
articles published on nanodentistry, and a few on its application in
orthodontics, none of them gives an overview of the various tests
performed to detect the physical and biological properties of the new
nanomaterials. This article provides an easy reference to a researcher
who is experimenting on application of nanoparticles. The review
concludes with an outlook of future scope of nanotechnology in
orthodontics.
Introduction
Revolutions in the field of science and technology
have given promising results in the field of material sciences and one
such advancement is nanotechnology. Nanotechnology, which concerns
structures at the Nano scale, is considered as a vital current
technology of the 21st century based on its economic and
scientific potential. Its application is being experimented in various
domains in orthodontics, from surface coatings to development of novel
materials.
What are nanoparticles?
British Standards Institution defines nanoparticles
as those particles in which all the fields or diameters are in the
nanoscale range. Whereas, nanomaterials are those material for which at
least one side or internal structure is in the nanoscale [1]. An
engineered nanoparticle may be defined as any intentionally produced
particle that has a characteristic dimension from 1 to 100 nm and has
properties that are not shared by non-nanoscale particles with the same
chemical composition [2].
What is nanotechnology?
Nanotechnology is the science of manipulating matter,
measured in the billionths of a nanometer, roughly the size of two or
three atoms [3].
What is nano dentistry?
It is the science and technology of maintaining
near-perfect oral health through the use of nanomaterials including
tissue engineering and nanorobotics [4].
Types of Nanoparticles
Nanoparticles are generally classified based on their
dimensionality, morphology, composition, uniformity, and agglomeration.
The various types of nanoparticles are Nano pores, Nanotubes, Quantum
dots, Nano shells, Dendrimers, Liposomes, Nano rods, Fullerenes, Nano
spheres, Nanowires, Nano belts, Nano rings and Nano capsules [5].
Following are some of the successfully employed nanoparticles in various
uses:
Silver
Silver nanoparticles have been found to be effective
against bacteria, viruses and other eukaryotes [6,7]. Successful
employment of these nanoparticles as antimicrobial agents
is being done in textile industries, for water treatment, in
cosmetics like in sunscreen lotions [8,9] and widely in dentistry
in fabrication of new materials like cements and resins etc. Green
synthesis of silver nanoparticles by plants such as Azadirachta
indica [10], Capsicum annuum [11] and [12] has also been
reported by various studies hence reducing their cytotoxicity.
Gold
Gold nanoparticles (AuNPs) have found application in
immunochemical studies for protein identification and are also
used for DNA detection and cancer diagnosis [13,14]. Nano
stenciled RGD gold patterns are being used for tissue engineering.
Alloy
The structural properties of alloy nanoparticles differ from
their bulk samples [15]. Silver flakes are widely used as silver
has the highest electrical conductivity among metal fillers and
their oxides have relatively improved conductivity [16]. The
properties of bimetallic alloy nanoparticles are influenced by
both metals and show better properties than ordinary metallic
NPs [17].
Magnetic
Magnetic nanoparticles like Fe3O4 (magnetite) and Fe2O3
(maghemite) have been actively studied for their possible use in
various fields including treatment of cancer, gene therapy, DNA
profiling, sorting and manipulation of stem cells, guided drug
delivery systems, and magnetic resonance imaging (MRI) [18].
Copper
Due to the antibacterial and antifungal activity along with
the catalytic, optical, electrical properties and application of
copper nanoparticles has been quite a focus in health–related
issues. Synthesis of nano-copper particles is mostly done in the
micro emulsion form.
Chitosan
It is a biopolymer derived by the deacetylation of chitin,
a natural polymer that occurs in exoskeleton of crustaceans.
Chitosan is a positively charged particle which is soluble in acidic
to neutral solution. These nanoparticles are being investigated
as a potential platform for local drug delivery.
Quarternary Ammonia Nanoparticles
Quarternary poly ethylene imine nanoparticles as
antimicrobials incorporated in composite resins have been
developed. The hydrophobic nature and the cationic surface
charge of these particles add on to their antimicrobial activity.
Zinc Compounds
These nanoparticles exhibit antibacterial, anti-corrosive,
antifungal and UV filtering properties. Low toxicity and good
biocompatibility make it suitable for biomedical usage. Nano
Zinc can decrease biofilm formation by inhibition of the active
transport and metabolism of sugars as well as disruption of
enzyme systems by displacement of magnesium ions essential
for enzymatic activity of the of dental biofilms [19].
Titanium Dioxide
Nanoparticles of this compound have been used in
biomaterials in order to induce antimicrobial properties.
Effective catalytic effect and other properties such as white color,
low toxicit, high stability and efficiency along with availability
and low cost have made these nanoparticles an appropriate
additive for use in dental materials [20].
Others
Nanoparticles of oxides under consideration for use include
those of silica, tin, copper and tungsten trioxide.
Uses of Nanoparticles in Dentistry
Nanoparticles have been successfully used in various forms
in dentistry from administering local anesthesia, simple cure of
dentinal hypersensitivity to diagnosis and cure of oral cancer.
Nano needles and Nano fibers have been employed for wound
dressings [21]. Nanoparticles due to their property of biocidal,
anti-adhesive, and delivery capabilities are being explored to
prevent the formation of biofilms within the oral cavity. As
nanoparticles possess a greater surface-to-volume ratio when
compared with non-nanoscale particles, they can interact more
efficiently with microbial membranes and provide considerably
larger surface area for antimicrobial activity. Metal NPs in the
size range of 1-10 nm have particularly shown the greatest
biocidal activity against bacteria. Nanoparticles can be used as
device coatings, as topically applied agents, and within dental
materials [22,23].
Nanoparticles of Silver have been identified to be considered
in dental resin composites as antimicrobial components. Low
percentages of silver – zinc antimicrobial zeolites added to
polymethyl methacrylate can be used for the reduction of
microbial contamination of tissue conditioners, acrylic resin
denture bases, and acrylic base plates of removable orthodontic
appliances [24]. Incorporation of silver zeolite nanoparticles
into mouth rinses and toothpastes has also been tested [25].
Small size of silver and zinc particles makes penetration through
cell membranes of microbe easier, thus affecting intracellular
processes resulting in higher reactivity and antimicrobial
activity [26].
Nanoparticles can also be used in various restorative dental
materials and procedures, including cavity liners, pit and fissure
sealants, cores and buildups, indirect restorations, cements
for crowns or orthodontic devices, provisional restorations,
endodontic sealers, and root canal posts [27]. Nanofillers integrated in vinylpolysiloxane have been seen to produce a
unique addition to siloxane impression materials that have
better flow with improved hydrophilic properties and enhanced
detail precision [28]. Mixing of alginate impression powders with
water containing silver hydrosol can be considered to create an
impression material with an antimicrobial property, reducing
microbial cross contamination to the poured stone model from
the infected impression [29].
Application of Nanotechnology in Orthodontics
Nano-coatings in arch wires and brackets to reduce friction
Friction is one of the major deterrents present in alignment
or retraction of teeth during orthodontic treatment. To conquer
over it one method is to apply higher forces, which might lead to
undesirable anchorage loss. The other alternatives are to vary
the wire size and shape, altering the bracket design or coating
the wire surfaces with different materials which may aid in
conquering sliding resistance. These coatings have been applied
either on bracket surface, or S.S. or NiTi wires. In the previous
years many researchers have tried using tungsten disulfide as a
surface lubricant. Naveh et al. [30] and Samorodnitzky et al. [31]
reported reduced friction after coating Nickel-Titanium (NiTi)
wires with nanoparticles of WS2 in the laboratory [30].
Similarly stainless steel wires have been coated with a
composite coating of Nickel-phosphorous and fullerene-like
nanoparticles of tungsten disulfide (WS2) placed by composite
electro less deposition [32]. Composite coatings of Co and
fullerene-like WS2 nanoparticles have also been tried [33]. WS2
nanoparticles have been incorporated to Ni–W–P alloy coating
and they not only reduced the coefficient of friction but also
helped in improving the corrosion resistance of the coating
further [34].
Considering possible toxicity of WS2, new self-lubricating
coatings, in which metals other than WS2 have been used. Wei
et al. [35] suggested use of Carbone Nitride (CNx) coatings on
stainless steel wires [35]. Similarly coatings of ZnO [36,37].
Inorganic fullerene like Molybdenum Disulfide nanoparticles
[38] and diamond like carbon coating and nitro carburizing
[39] have been suggested. The nanostructured DLC coating also
provided excellent corrosion resistance and good elasticity when
coated on S.S. wires.
Fabrication of hollow wires
Hollow wires are wires coated with NiTi/Ni-TiO2 composite
nanoparticles via the synthesis method called ultrasonic spray
pyrolysis (USP). The precursor solution for the synthesis of
spherical NiTi particles is prepared from an orthodontic wire
with a chemical composition of Ni (amount fraction x = 51.46 %)
and Ti (x = 48.54 %). A textile or polymer fiber is coated with NiTi
nanoparticles via electrospinning and then the fiber is removed
to produce a hollow wire for orthodontic purposes. This wire
could potentially have the shape-memory and superelasticity properties, while possibly reducing the material needed for
the wire production. However with the current selection of the
precursors, reaction gas and collection medium, it was difficult to
obtain pure NiTi particles, which were desired. For this reason,
further investigation of different precursor solutions, gases and
collection media needs to be conducted [40].
Orthodontic brackets
A new material which contained polysulfone embedded
with hard alumina nanoparticles was developed in the year
2012 by UC3M for making orthodontic brackets. The material
innovated had the properties of strength, reduced friction and
biocompatibility while maintaining the transparency of the
bracket [41].
Nanoparticles application as antimicrobial agent
White spot lesions and caries are common problems
encountered while undergoing orthodontic treatment due to
plaque accumulation around brackets. Nitrogen doped Titanium
dioxide (TiO2), Silver (Ag), Gold (Au) , Silica (SiO2) Copper
(Cu/CuO) and ZnO nanoparticles have been coated on either
brackets or added to cements and bonding agents to reduce the
demineralization produced as a result of orthodontic treatment.
Nitrogen doped titanium dioxide (TiO2) brackets:
Orthodontic brackets have been coated with nitrogen doped
titanium dioxide. The activation of Nitrogen doped Titanium
dioxide leads to the formation of OH. Free radicals, superoxide
ions (O2), peroxyl radicals (HO2) and hydrogen peroxide (H2O2).
These chemicals, through a series of oxidation reactions, react
with biological molecules such as lipids, proteins, enzymes and
nucleic acids, damage biological cell structures, but also exert
antimicrobial activity. Limitation of this study is that long-term
clinical performance and safety of the newly modified bracket
surfaces as well as the effects on the bond strength to teeth are
missing [42]. TiO2 nanoparticles of size 21±5nm have also been
blended to light cure orthodontic composite paste (Transbond
XT) in 1, 2, and 3% .All the three concentrations had similar
antibacterial effects [43].
Fluoroapatite, fluorohydroxyapatite or hydroxyapatite
NPs: Resin modified GIC has been improved by incorporating
nano-sized fluoroapatite (NFA) or fluorohydroxyapatite (NFHA)
particles at 25% concentration; however, this was at the cost
of significant reduction in shear bond strength. The fluoride
release nearly tripled after 70 days [44]. Nano-hydroxyapatite
(Nano-HA) has also been added to orthodontic banding cement
to prevent microleakage. This study assessed the microleakage
under orthodontic bands by the methylene blue dye penetration
method after 60 days [45].
Chitosan nanoparticles: Different concentrations of ZnONPs
and CS-NPs mixture: 1%, 5% and 10% (1:1 w/w) were
added to resin composite to induce antibacterial activity .It was
found that Zinc NP when mixed with Chitosan NP in the ratio 10% (w/w) significantly induces antibacterial property higher
than other groups [46].
Silver nanoparticles: Silver NPs have been added to
composite adhesive containing silica nanofillers. Addition
of silver NPs significantly reduced the adhesion of cariogenic
streptococci to orthodontic adhesive relative to conventional
adhesives, without compromising physical properties (shear
bond strength). To increase antimicrobial activities, various
concentrations of silver nanoparticles (diameter < 5 nm) have
been added to the composite adhesive: 0 ppm, 250 ppm, and
500 ppm [23]. Silver and HA nanoparticles have also been
added to the primer of Transbond XT in 1%, 5% and 10%
silver concentrations. It was found that incorporation of silver/
HA nanoparticles in 5% and 1% concentration maintains and
increases the Shear bond strength of orthodontic adhesives,
respectively, whereas increasing the amount of particles to 10%
has an undesirable effect when compared to the control group
[47].
Nanosilver coating process has been applied to orthodontic
brackets placed in rat. Dental plaque, mucosal vestibular
smears, saliva, and blood samples were collected from rats at
various days. It is suggested that nanosilver coated orthodontic
brackets, as an antibacterial agent without patient compliance,
could be helpful for the prevention of white spot lesions during
fixed orthodontic treatment. Since bacterial infection has been
identified as one of the major causes of titanium implant
failures, a novel antibiotic vehicle composite, TiO2NT–PSPMA,
has been synthesized via atom transfer radical polymerization;
this method improved the local antibiotic concentration and
prolonged its sustainable release by loading larger amounts of
antibiotic into Titanium nanotubes (TiO2 NTs) arrayed on Ti
implants. Ag nanoparticles (NPs) were loaded into TiO2 NTs with
the assistance of the ionic polymer 3-sulfopropyl methacrylate
potassium salt (PSPMA). This composite increased the storage of
Ag NPs by employing nanotubes and using PSPMA to trap larger
amounts Ag NPs. This experiment showed that the composite had
a dose-dependent cell proliferation by 3-(4, 5-dimethylthiazol-2-
yl)-2, 5-diphenyltetrazolium bromide (MTT), indicating that the
composite perhaps could be used in future to prevent implant
infection [48]. Silver nanoparticles have been successfully
added to PMMA to produce an antimicrobial resin without
compromising on their physical properties. However, their long
term effects on tissues need to be verified [49,50].
Copper: Copper NPs have been added to orthodontic adhesive
at 0.0100 wt%, 0.0075 wt%, and 0.0050 wt%. Significantly higher
bond strength was obtained with the orthodontic adhesive that
included 0.0100 wt% of copper NPs [51].
TiO2, Sio2 or Silver NPs to acrylic resins: TiO2, SiO2 or silver
NPs have been added to Cold-cure acrylic resins that are mainly
made of polymethyl methacrylate (PMMA). The limitations with
these studies are that some did not assess the antibacterial
or safety of the NP-incorporated acrylic materials [52,53] or
assessed the biocompatibility over a short period of time (24–72 h) [51,54]. The NP size may also affect the cytotoxicity and
immunological response.
Zinc oxide: Zinc oxide has been added to light cured Resin
modified glass ionomer to create mixtures of 13% ZnO and
23.1% Zinc oxide. It has been observed that as the concentration
of Zinc oxide increases, antimicrobial activity significantly
increases. Antimicrobial activity of Zinc oxide lasts for at least
1 month, albeit at lesser levels. It was also observed that as
the concentration of zinc oxide increased, shear bond strength
decreased. Future studies should evaluate more refined methods
of adding zinc oxide in order to have less impact on the physical
properties of the bonding agent. Further clinical studies are
needed to assess the capabilities of zinc oxide as an intraoral
antimicrobial agent [55]. Combined effect of Zinc oxide and CuO
has also been studied and it has been observed that CuO and ZnOCuO
nanoparticles coated brackets have better antimicrobial
effect on S.mutans than brackets coated with Zinc oxide or CuO
alone [56].
Fluoride releasing nanoparticles: Fluoride releasing
and enamel demineralization inhibition capacity of fluoridereleasing
nano filled cement around orthodontic brackets has
been evaluated using an artificial caries biofilm model. 4 groups:
non-fluoride-releasing microfilled composite, fluoride-releasing
microfilled composite, resin-modified glass ionomer cement
(RMGI), and fluoride-releasing nanofilled composite (FN) were
tested. Under the cariogenic exposure condition of this study, the
fluoride-releasing nanofilled material had similar performance
to fluoride-releasing microfilled materials. The presence of
nanofillers in the fluoride releasing materials studied did not
promote further benefits against caries lesion development
around brackets and presented inferior demineralization
inhibition than the resin modified glass ionomer material [57].
Quaternary ammonium monomer dimethyl aminododecyl
methacrylate (DMADDM): In 2014, DMADDM, a recentlysynthesized
antibacterial monomer, was incorporated into
orthodontic cement at 0%, 1.5%, 3% and 5% mass fractions and
then the bond strength of brackets to enamel was measured. A
microcosm biofilm model was used to measure metabolic activity,
lactic acid production, and colony-forming units. DMADDMcontaining
orthodontic bracket cement possessed a strong
antimicrobial activity when incorporating 3% of DMADDM. The
anti-biofilm potency increased with increasing the DMADDM
mass fraction; however, the enamel bond strength had a slight
decrease at 5% DMADDM [58].
Use of Nanoparticles in Tissue Engineering
Nano-stenciled rgd-gold patterns
An experiment was done to analyze how restricting the size
of cell-matrix adhesions affects cell morphology and behavior.
Cultured fibroblasts adhere to extracellular substrates by means
of cell-matrix adhesions that are assembled in a hierarchical
way, thereby gaining in protein complexity and size. Using a
nanostencil technique, culture substrates were patterned with gold squares of a width and spacing between 250 nm and 2
μm. The gold was functionalized with RGD peptide as ligand for
cellular integrins, and mouse embryo fibroblasts were plated.
Limiting the length of cell-matrix adhesions to 500 nm or less
disturbed the maturation of vinculin-positive focal complexes
into focal contacts and fibrillar adhesions, as indicated by poor
recruitment of α5-integrin. It was found that on sub-micrometer
patterns, fibroblasts spread extensively, but did not polarize.
Instead, they formed excessive numbers of lamellipodia and a
fine actin meshwork without stress fibers. Moreover, these cells
showed aberrant fibronectin fibrillogenesis, and their speed
of directed migration was reduced significantly compared to
fibroblasts on 2 μm square patterns. Interference with RhoA/
ROCK signaling eliminated the pattern-dependent differences
in cell morphology. Our results indicate that manipulating the
maturation of cell-matrix adhesions by nanopatterned surfaces
allows to influence morphology, actin dynamics, migration and
ECM assembly of adhering fibroblasts. Thus in the future, the
nanostencil method may offer new possibilities to control more
precisely the interaction of mesenchymal cells with implant
surfaces, and to influence their differentiation around the
implant [59].
Nanoclay reinforced magnesium substituted E-Tcp
Advances in the field of nanotechnology presented a
wide range of solutions to biological problems of high rate of
microimplant failure. A nanocoating of nanoclay reinforced
magnesium substituted E-TCP was placed on titanium surface to
enhance the stability of orthodontic miniscrews. The nanoclay
used is Na+-montmorillonite (“Cloisite Na+”) powder (Southern
Clay Products, TX, USA). The nanoclay suspension was prepared
by dissolving clay powder in DI water under vigorous stirring for
1 week prior to use [60].
Nanosized hydroxyapatite paste/scaffolds
Biomimetically synthesized nanosized hydroxyapatite
particles have been converted into an injectable paste using a
neutral phosphate buffer. Synthesized system manifested a self
setting behavior at 37°C in 20 min and revealed a macroporous
self assembled microstructure. Stability of the injectable
hydroxyapatite has been confirmed in aqueous medium as well
as in human blood. These hydroxyapatite pastes can be used to
fill defects in damaged bone due to any cause [61].
Titanium nanotubes with embedded silver oxide nanoparticles as biomedical coating
TiO2 nanotube (NT) arrays have been found to significantly
enhance the functions of many cell types including osteoblasts
thus having promising applications in orthopedics, orthodontics,
as well as other biomedical fields. TiO2 NT arrays with Ag2O
nanoparticles embedded in the nanotube wall (NT-Ag2O
arrays) were prepared on titanium (Ti) by TiAg magnetron
sputtering and anodization. Well-defined NT arrays containing
Ag concentrations in a wide range from 0 to 15 % were formed.
Crystallized Ag2O nanoparticles with diameters ranging from 5 nm to 20 nm were embedded in the amorphous TiO2 nanotube
wall and this unique structure lead to controlled release of Ag
that generated adequate antibacterial activity without showing
cytotoxicity. The NT- Ag2O arrays can effectively kill Escherichia
coli and Staphylococcus aureus even after immersion for 28 days,
demonstrating the long lasting antibacterial ability. Furthermore,
the NT- Ag2O arrays have no appreciable influence on the
osteoblast viability, proliferation, and differentiation compared
to the Ag free TiO2 NT arrays. Ag incorporation even shows some
favorable effects on promoting cell spreading and can be used as
a biomedical coating on devices [62].
Nano-Materials as Nanofillers in Orthodontics
Nano-sized filler particles have been incorporated into the
composite matrix and glass ionomer cements. Nanofillers are of
two types: nanoclusters and nanoparticles [63]. Nanofillers can
be prepared by techniques, such as flame pyrolysis, flame spray
pyrolysis, and sol-gel processes. The addition of fillers reduced
size has capacitated filler load enhancement thus reducing
polymerization shrinkage and improving mechanical properties
of strength. Various studies have tested the bond strength
of nanocomposites and nanoionomers and have concluded
that they can be used for orthodontic bonding [63-66]. Silica
nanosized filler particles (10 wt%, particle diameter < 7 nm)
have also been added to orthodontic adhesives [23]. Titanium
dioxide and zirconia are particularly useful nanofillers, as they
have very high refractive indices, and will require less weight
of material than a lower refractive index material to match the
refractive indices appropriately [67]. Nanozirconia has also been
used in ionomer cements and provides for improved properties,
including enhanced aesthetics (e.g. low visual opacity), polish
retention, and radiopacity as compared to previously known glass
ionomer compositions. The nanozirconia is surface modified
with silanes to aid in the incorporation of the nanozirconia into
ionomer compositions [68].
Enamel Remineralizing Agents
Nano particles have been used not only as antimicrobial
agents but as agents for remineralization of decalcified enamel.
Nano-hydroxyapatite has been introduced as nanotechnological
advancement in the products for the remineralization of
enamel and has been developed as a paste. Medeiros et al. [69]
concluded that calcium nanophosphate forms a protective layer
on the enamel surface and provides protection against erosion.
Calcium nanophosphate crystals which are smaller than 100
nm, lead to improved bioactivity of the product, resulting from
the increase in surface area and wet ability of HA nanoparticles.
Calcium, phosphate and fluoride ions are released and organized
on fluoroapatite and CaF2 on demineralized tooth surface. In a
comparative study by Carvalho et al. [70] on the effect of calcium
nanophosphate and CCP-APP paste, it was concluded that calcium
nanophosphate is a better remineralizing agent for eroded
enamel surfaces. Thus, calcium nanophosphate could be used as
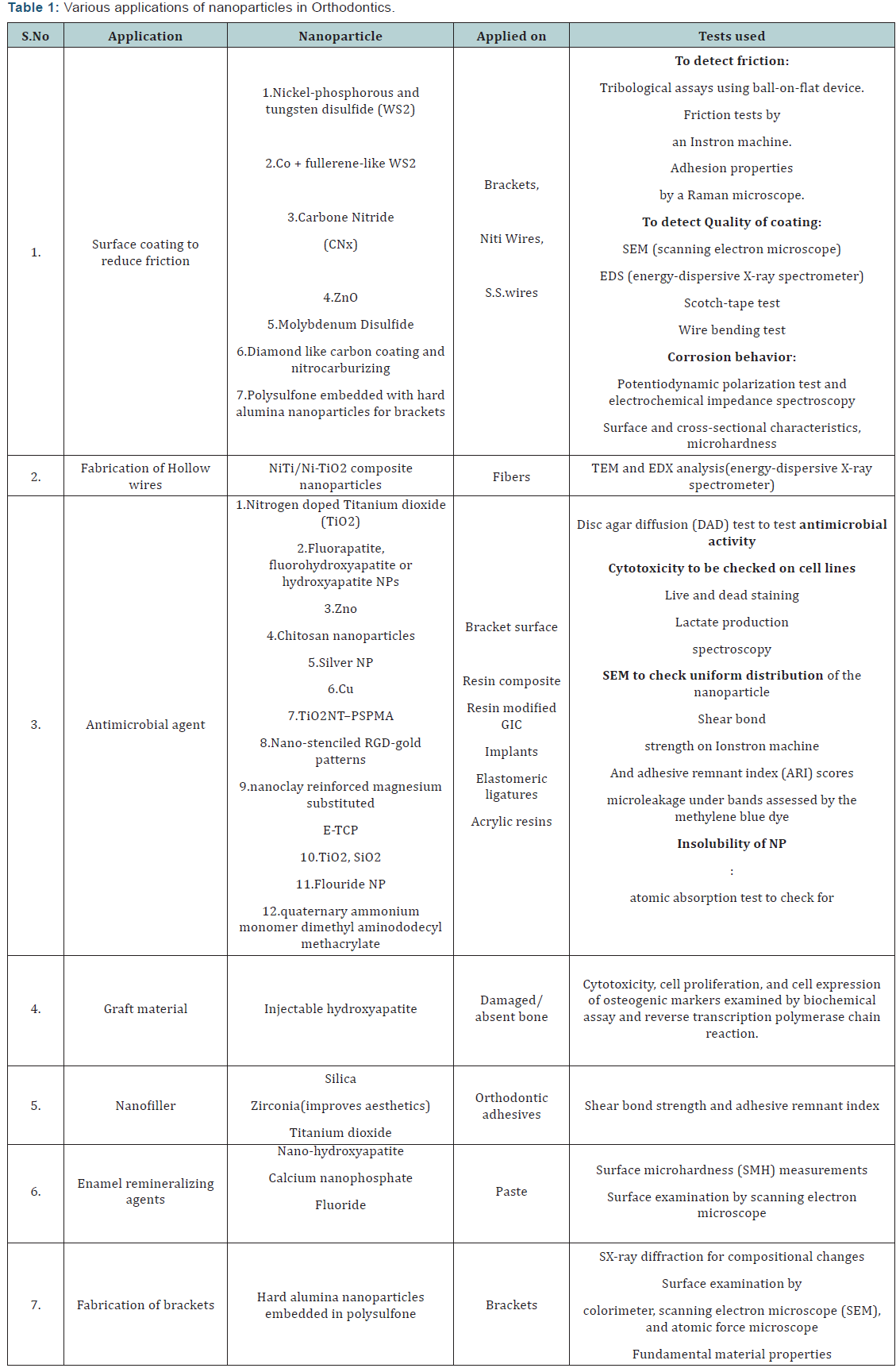
a remineralizing agent after debonding of orthodontic brackets [70]. Various applications of nanoparticles in orthodontics are
summarized in (Table 1).
Future Applications of Nanotechnology
Nanorobots in orthodontics
Nanorobotics centers are self-sufficient machines which
are functional at the nanoscale. The nanorobot design consists
of a biocompatible glycocalyx-coated diamondoid material
with molecular sorting rotors and a robot arm (telescoping
manipulator) [71]. Different nanorobot molecule types are
distinguished by a series of chemotactic sensors and their
functioning is controlled by a stimulator. Nanorobots may be
used for manipulation of tissues directly at nano level and
research has begun on the use of nanorobotics for medical
applications like drug delivery, management of aneurysms
and tumors. The theory of use of such nanorobots could be
extended to dentistry and orthodontics in distant future, where
nanorobots with specific motility mechanisms would navigate
through periodontium to remodel it directly allowing accelerated
orthodontic tooth movement.
Nanoindenter
A nanoindenter coupled with atomic force microscope
(AFM) is used to evaluate nanoscale surface characteristics of
bio-materials. They have also been used to evaluate mechanical
properties such as hardness, elastic modulus, yield strength,
fracture toughness, scratch hardness and wear properties by
nano indentation studies [72].
Bio Mems/nems for orthodontic tooth movement
Biomedical Microelectromechanical systems (Bio MEMS)
can be defined as the science and technology of operating at the
microscale level for biological and biomedical applications, which
may or may not include any electronic or mechanical functions.
The MEMS micromachined elements include gears, motors and
actuators with linear and rotary motion for applications to
biological systems. Nanoelectromechanical systems (NEMS) are
devices integrating electrical and mechanical functionality on
the nanoscale level. It has been proposed that microfabricated
biocatalytic fuel cells (enzyme batteries) can be used to generate
electricity to aid orthodontic tooth movement. An enzymatic microbattery when placed on the gingiva near the alveolar bone
might be a possible electrical power source for accelerating
orthodontic tooth movement. However, there are several issues
like soft tissue biocompatibility, effect of food with different
temperature and pH range on the output of such microfabricated
enzyme battery that need to be addressed. It is expected that
the MEMS/NEMS based system will be applied over the next few
years to develop biocompatible powerful biofuel cells, which can
be safely implanted in the alveolus of the maxilla or mandible to
enhance orthodontic tooth movement [73,74].
Nano LIPUS devices
Ultrasound is a form of mechanical energy that is transmitted
through and into biological tissues as an acoustic pressure wave
at frequencies above the limit of human hearing, is used widely in
medicine as a therapeutic, operative, and diagnostic tool [75,76].
LIPUS has been reported to enhance bone growth into titanium
porous–coated implants [77] and bone healing after fracture
[78,79] and after mandibular distraction osteogenesis [80]
and has also stimulated mandibular cartilaginous growth [81].
Another application of this technique is to reduce root resorption
during orthodontic treatment. Based on their observation that
LIPUS can promote dental tissue formation in rabbits, El Bialy
et al. [82] concluded that it may be used to treat root resorption.
The unit will be easily mounted on a bracket or even a plastic
removable crown. An energy sensor can also be used that will
ensure the LIPUS power is reaching the target area of the teeth
roots within the bone.
Smart brackets with nanomechanical sensors
The concept of a smart bracket with integrated sensor
system for 3D force and moment measurement has recently
been published. Nanomechanical sensors can be fabricated and
be incorporated into the base of orthodontic brackets in order to
provide real-time feedback about the applied orthodontic forces.
This real-time feedback allows the orthodontist to adjust the
applied force to be within a biological range to efficiently move
teeth with minimal side effects [83,84] (Table 1).
Conclusion
A lot of research is being focused on the application of
nanotechnology in orthodontics. Though much of the research
has taken place in the labs, gradually in vivo studies are making
their way. Biosafety of nanoparticles and materials is a subject of
concern, demanding focus on further studies of the toxic effects
of nano-particles to ensure their ethical usage in the oral cavity.
The future in orthodontic treatment will benefit enormously
through nanotechnology should all the current attempts succeed
to its clinical application at a reasonable cost to the orthodontist
and patients.
For more Open Access Journals in Juniper Publishers please
click on: https://juniperpublishers.com
For more articles in Open Access Journal of
Dentistry & Oral Health please click on:

Comments
Post a Comment