Guided Tissue Regeneration using an Equine Bio-absorbable Collagen Membrane with or without Equine Bone Graft in the Treatment of Intrabony Defects in Patients with Aggressive Periodontitis Results of 18 month- Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DENTISTRY & ORAL HEALTH
Guided
Tissue Regeneration using an Equine Bio-absorbable Collagen Membrane with or
without Equine Bone Graft in the Treatment of Intrabony Defects in Patients
with Aggressive Periodontitis Results of 18 month
Authored by Mahmoud Helmy Belal
Abstract
Background:Successful
periodontal regeneration is considered a gold standard for
periodontists. Several GTR materials and bone grafts have been attempted
but showed variable success rates.
Objective:The present randomized
clinical and radiographic study was undertaken to compare the
effectiveness of guided tissue regeneration (GTR) by using equine
bioabsorbable collagen membrane with or without equine bone graft in the
treatment of periodontal intrabony defects affected by aggressive
periodontitis.
Materials and Methods: Ten
systemically healthy patients with 20 periodontal intrabony defects were
enrolled as a split mouth design. These defects were affected with
aggressive periodontitis. The recorded measurements included plaque
index, gingival bleeding index, gingival recession, PPD, clinical
attachment level, radiographic defect depth and radiographic density.
The defects were randomly distributed either as a control group (equine
bioabsorbable collagen membrane alone) or a test group (equine
bioabsorbable collagen membrane combined with equine bone graft).
Results: The inter- &
intra-group comparison showed no significant differences between studied
groups nor within each group for these clinical parameters (PI, BI
& GR), except only gingival recession within group 1. On contrary,
the inter- & intra-group comparison showed significant differences
between studied groups and within each one for these parameters (PPD,
CAL, RDD & RD).
At 18-month examination, PPD reduction was
significantly greater in GTR + bone graft group (3.80±1.33 mm) compared
with GTR group (2.60±1.57 mm), and clinical attachment level gain were
3.60±1.15 and 2.20±1.26 respectively. Radiographic DD reduction was
similarly greater in GTR + bone graft group (3.30 ± 0.84 mm) compared
with GTR group (2.40±1.09 mm). Also, the change in the radiographic
density indicated a significant greater gain of mean gray level as
(19.90 ± 16.00) in group 2 whereas 7.10±10.65 in group 1.
Conclusion: Use of GTR
bioresorbable membrane with bone graft showed significant improved
outcomes when compared to use of bioresorbable membrane in treating
aggressive periodontitis. However, the studied groups showed significant
improvement within each group when baseline & 18 month data were
compared.
Keywords: Aggressive Periodontitis; Bone Graft; Guided Tissue Regeneration; Intrabony DefectAbbreviations: AP: Aggressive Periodontitis; GTR: Guided Tissue Regeneration; CAL: Clinical Attachment Level; PPD: Probing Pocket Depth; GR: Gingival Recession; BI: Bleeding Index; PI: Plaque Index; RD: Radiographic Density; RDD: Radiographic Defect Depth; CBR: Crestal Bone Resorption; AC: Alveolar Crest; CEJ: Cementoenamel Junction; BD: Base of the Defect
Introduction
The aim of successful periodontal therapy is a trial
to arrest inflammatory process, suppress microbial growth, control
infection and attempt to restore the tissues destroyed due to
periodontal disease. Different regenerative techniques may be used for
such purposes, but the treatment or elimination of the infection should
be the most important goal especially in patients having aggressive
periodontitis [1,2].
Aggressive periodontitis (AP) is characterized by
severe periodontal destruction within a relatively short period of time.
It has some etiologic agents capable of causing clinically detectable
diseased levels that may negatively hazard oral healthy condition
and alter daily life style of such patients. AP has less occurrence
among periodontitis patients and few studies have evaluated
some treatment protocols [3].
The predictable complete periodontal regeneration remains
a major goal in the planned therapy. Despite several procedures
such as usage of guided tissue regeneration (GTR), grafting
materials, growth factors and/or host modulating agents have
been attempted, the outcomes are not always predictable [4-6].
However, there is a great variation caused by many factors, for
example; type of periodontitis, patient characteristics, anatomy
of defect site and the surgical intervention [7]. The treatment of
aggressive type of periodontitis has a great challenge to many
clinicians.
In general, the most successful documentation of
periodontal regeneration is GTR since it acts as an effective
principal therapy for the treatment of different anatomic defects
associated with periodontitis [5]. Some earlier animal [8,9] and
human studies [10,11] indicated a predictable reconstruction
of the periodontium by using either non-bioabsorbable or
bioabsorbable membranes [12]. However, bioabsorbable
GTR membranes were developed to avoid the second surgery
needed to retrieve the non-resorbable barrier [13-16]. These
bioabsorbable devices have two main products, natural (collagen
membrane) and synthetic (copolymers) like Guidor, Vicryl
periodontal mesh, Resolut and Atrisorb GTR barriers.
Use of GTR through the use of equine barrier and equine
bone graft material showed a favorable clinical outcome and an
effective periodontal therapy in the regenerative treatment of
intrabony defects [17]. Also, these equine collagen membranes
and equine bone acts as an effective therapy for guided bone
regeneration in the treatment of bone defect consequent to
removal of periapical cyst in clinical & histological report [18].
Some previous literature reports [13-16] were found,
in which the efficacy of bioabsorbable membranes alone or
combined with graft materials were evaluated and compared
for regenerative purposes. However, to our knowledge, there
are no available studies comparing the efficacy of using an
equine bioabsorbable collagen barrier (Biocollagen®) alone or
combined with equine graft (Bio-Gen®), in treating intrabony
defects of aggressive periodontitis.
Subjects and Methods
Inclusion criteria
- Aggressive periodontitis.
- Intrabony periodontal defect sites with probing pocket depth > 5mm, as assessed by clinical and radiographic evaluation.
- Radiographic evidence of vertical/angular bone loss.
- Age ranged from 17 to 37 years.
- Good general health.
Exclusion criteria
- Periodontal treatment received during the last 6 months at least.
- Hopeless teeth or evident grade-III mobile teeth.
- Any relevant systemic diseases.
- Smokers and/or alcoholics.
- Pregnancy and/or lactation for female patients.
- Hypersensitivity to any of the tested research materials.
Study Design
Ten patients (seven males & three females) were selected
to be enrolled in this study and gathered from the out-patient
clinic of the Department of Oral Medicine, Periodontology, Oral
Diagnosis & Radiology. Verbal and written informed consent
forms were obtained from all subjects and an ethical clearance
was also get from the institution.
Ten patients with bilateral intrabony periodontal defects
were selected. Thus, a total of twenty affected sites were chosen
as noticed primarily on the radiographs and confirmed clinically
as well as within the reconstructive surgical intervention. These
defects were distributed into two groups as follows:-
- Group 1 (the control group): Ten sites received flap debridement followed by the application of bioabsorbable equine collagen membrane* (Biocollagen®).
- Group 2 (the test group): Ten sites received flap debridement followed by the application of the same bioabsorbable collagen membrane but combined with equine bone graft material as xenograft† (Bio-Gen®).
Primary Assessment and Patient’s Preparation
Patients were subjected to pre-surgical, clinical and
radiographic interpretation. The patients completed a thorough
plaque control regimen and a strict oral hygiene instruction.
Full-mouth phase I therapy was done using periodontal Gracey
curettes# and an ultrasonic apparatus$. The reevaluation period
was determined according to each individual response, with an
average period of about 6 weeks. Thereafter, a treatment plan
was defined and an additional non-surgical therapy and/or
dental extractions were done whenever needed. However, the
surgical intervention were started when the subject’s plaque
and gingival index had achieved at least 20% levels, according to
instructions of some previous reports [19-21].
Clinical Measurements
Plaque index (PI), gingival bleeding index (BI), gingival
recession (GR); probing pocket depth (PPD) and clinical attachment level (CAL) were recorded. An acrylic stent with
reference points was used to localize the measurement sites
at baseline and 18 months postoperatively. The periodontal
measurements were recorded using a graduated William’s
periodontal probe‡. Tooth mobility was also graded [22], scored
and evaluated.
Surgical Procedures
Access to the defects were done using full-thickness flaps
and sulcular incisions through the bottom of the crevice,
extending mesial and distal to the adjacent teeth and including
the flap papillae. No releasing incisions needed on either sides of
the flap. Granulomatous tissues were curetted and thorough root
planning was performed. EDTA with a concentration of 24%, at
neutral pH, was used then washed with saline irrigation.
Randomly, using a coin, one defect treated with equine
bioabsorbable collagen membrane as a control group whereas
the other defect was treated with the same collagen membrane
but combined with equine bone graft (the test group). Graft
material was soaked with sterile saline and condensed gently in
the defects to the adjacent crestal walls. The collagen membrane
was adapted to obtain precise application to the interproximal
area of the affected site. The membrane was then adjusted to
completely cover the defect, overlapping at least 2-3 mm of
the residual bone and sutured adequately with bioabsorbable
sutures. Flaps were repositioned coronally during wound
suturing, if required. The surgical site was covered with a noneugenol
periodontal dressing% on the buccal and lingual aspects.
Post Operative Care
Chlorhexidine digluconate mouth rinse (0.12%) was used
as two times daily for one month postoperatively. Amoxicillin
trihydrate 875 mg/clavulanic acid as potassium salt 125 mg
(Hibiotic 1gm film coated tablets, Amoun Pharmaceutical Co. SAE.,
El-Obour city, Cairo, Egypt), was prescribed as two times daily for
10 days. Also, a non-steroidal anti-inflammatory drug (Ibuprofen
600 mg, “Brufen granules”, effervescent sachets, Kahira Pharm.
& Chem. Ind. Co. under license of Abbott Laboratories-USA)
was also prescribed twice per day, for 7 days. In addition, antiprotozoal
drug (Secnidazole 1gm, Nitroimidazole derivative,
Cipazole forte, SIGMA Pharmaceutical Industries-Egypt, with
cooperation of Queen Pharm international) was prescribed two
tablets (each tablet contains Secnidazole 1gm) as a single dose
(one day treatment) during meal.
Postoperative care was performed weekly in the first month,
monthly up to six months and then every 3 months. In cases
of membrane exposure, Doxycycline 100 mg was prescribed 2
times per one day then once for 5-7 days, and the surgical sites
were carefully cleaned with a cotton swab soaked with 0.12%
chlorhexidine digluconate two times daily.
Assessment of radiographic parameters and radiographic interpretation
Standardized intra-oral periapical radiographs (Kodak
X-ray film, USA) were obtained at baseline and 18 months
postoperatively. These radiographs were taken using long cone/
extension cone paralleling technique with a positioning device
mounted on a roentgen machine, operating at 70 Kilo Voltage
Power.
Radiographs were scanned using a digital scanner at an
input of 300 dpi and 100% scale, then they were analyzed using
a software@. The images had 768 × 512 pixels and 256 gray scale
level. The alignment of images, in the pairs of radiographs, was
applied to correct small geometric misalignments. Gray level was
then calibrated to indicate changes in the radiographic density
(RD).
In addition, the following measurements were obtained
in millimeters: distance from cementoenamel junction (CEJ)
to base of the defect (BD) and from CEJ to alveolar crest (AC).
The differences between baseline and 18-month postoperative
values for CEJ–BD indicated the change in the radiographic defect
depth (RDD), whereas the differences for CEJ–AC suggested the
possible occurrence of crestal bone resorption (CBR).
Statistical Analysis
The present study had parametric variables. Thus, student’s
paired t-test was used to compare the changes in the data from
baseline up to 18 months postoperatively within each treatment
group. On the other hand, the intergroup comparison was
accomplished by independent sample t-test. A ‘P’ value of 0.05
or less was considered statistically significant.
Results
Using student’s paired t-test, the intra-group comparison
showed comparable outcomes in the experimental groups
regarding plaque index, gingival bleeding index as well as
gingival recession, when comparing baseline scores to 18 months
postoperative data. This is because there were no statistically
significant differences noticed for these parameters, except only
gingival recession within group 1 where P value recorded as
0.04. In addition, using independent sample t-test for intergroup
comparison there were no statistically significant differences
found between the two groups for these clinical parameters (PI,
BI & GR). (Table 1).
On the other hand, by using student’s paired t-test,
statistically significant differences were found when comparison
was done from baseline up to 18 month postoperatively within
both groups (1 & 2) regarding PPD, CAL, RDD & RD. Furthermore,
using independent sample t-test for intergroup comparison there
were also statistically significant differences found between the
two groups for these clinical and radiographic parameters (PPD,CAL, RDD & RD) (Table 1).

The probing depth is reduced from (7.80±1.75 mm) at
baseline to (5.20±1.40 mm) at 18 month with a mean difference
of (2.60±1.57 mm) in group 1, whereas from (7.90±1.66 mm)
to (4.10±.99 mm) with a mean difference of (3.80±1.33 mm)
in group 2. This was statistically significant (P = 0.000). The
intergroup comparison indicated that the difference was
statistically significant with a P value of 0.05 (Table 1).
The clinical attachment level is changed from (8.60±1.26
mm) at baseline to (6.40±1.26 mm) at 18 month postoperative
with a mean CAL gain of (2.20±1.26 mm) in group 1, whereas
from (8.60±1.35 mm) to (5.00±.94 mm) with a mean CAL gain of
(3.60±1.15 mm) in group 2. This was statistically significant (P =
0.000). The intergroup comparison indicated that the difference
was statistically significant with a P value of 0.01 (Table 1).
The radiographic defect depth was reduced from (5.90±1.10
mm) at baseline to (3.50±1.08 mm) at 18 month postoperative
with a mean difference of (2.40±1.09 mm) in group 1, whereas
from (5.90±.99 mm) to (2.60±.70 mm) with a mean difference of
(3.30±0.84 mm) in group 2. This was statistically significant (P
= 0.000).
The radiographic density was changed from (92.40±12.38)
at baseline to (99.50±10.91) at 18 month postoperative with
a mean difference of (7.10±10.65) in group 1, whereas from
(94.10±14.32) to (114±17.68) with a mean gain of (19.90±16.00)
in group 2. This was statistically significant (P = 0.000 & 0.03
respectively). In addition, the intergroup comparison regarding
both RDD and RD showed that the difference between the
studied groups was statistically significant with a P value of 0.04
(Table 1).
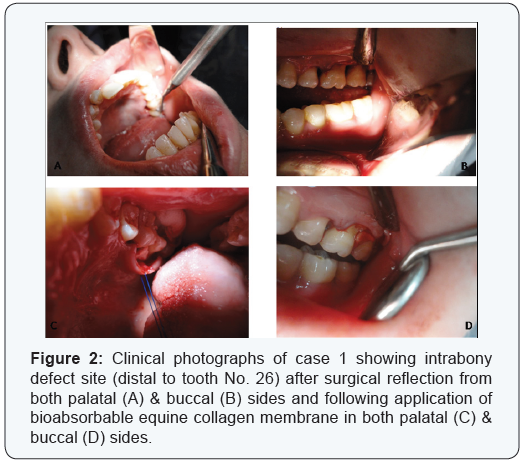
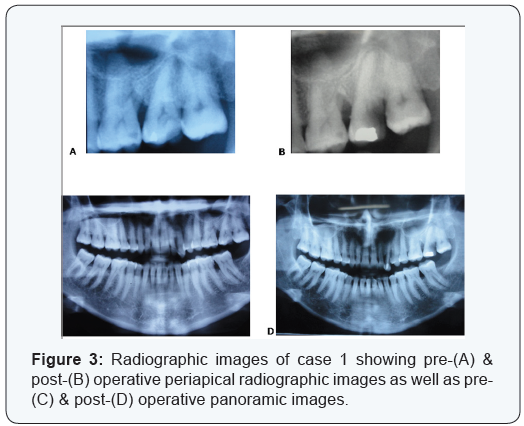
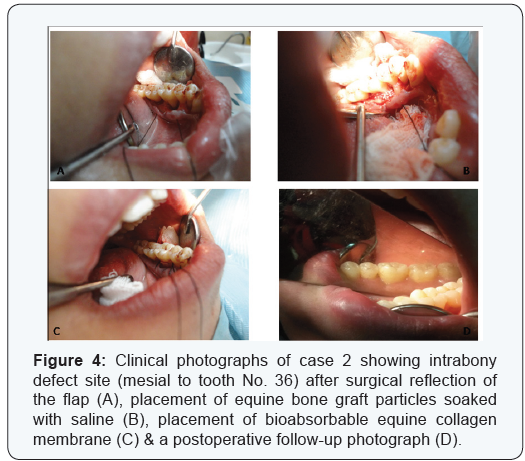
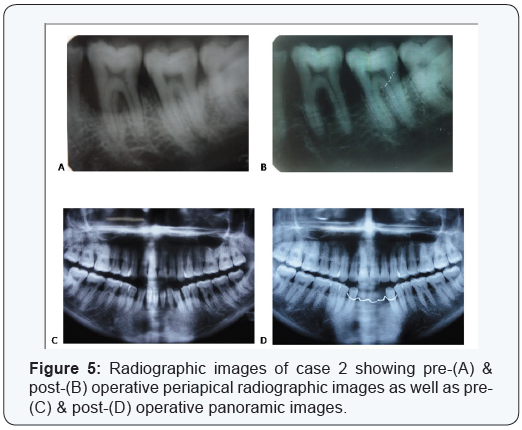
Two cases are presented in figures from 2 to 5 with clinical
photographs during surgical intervention and pre-& postoperative
radiographic images. Case 1 is presented in figures (
1 & 3) and was treated by using bio absorbable equine collagen
membrane, for covering and treatment of selected intrabony
defect site between teeth No. 26 & 27 as mainly distal to tooth
No. 26. Case 2 is presented in ( figures 4 & 5 ) and was treated by
the application of the same bio absorbable collagen membrane
but in combination with equine bone as xenograft material, for
covering and treatment of selected intrabony defect site between
teeth No. 35 & 36 as mainly mesial to tooth No. 36.

Discussion
It has been established in the previous literature [10,23] that
the exclusion of epithelial and gingival connective tissue cells by
using GTR barriers is important for periodontal wound healing,
in order to achieve regeneration of the attachment apparatus.
However, the non-restorable barriers have some disadvantages,
such as higher cost, membrane exposure, need of a second
intervention for their retrieval, complexity and bacterial
accumulation [24,25]. Several restorable barriers are therefore
developed to decrease such drawbacks. These are preferable and
widely used for guided tissue and/or bone regeneration [26,27].
Collagen membranes are selected frequently as restorable
barriers, especially they possess some advantageous properties.
These are a low toxicity due to a low immune response, the
ability of collagen to reconstitute into the natural tissues and
to enhance cell growth and attachment [28,29]. In addition,
collagen membranes are absorbed quickly to provide the needed
integrity during regenerative process.
Bone grafts are used to treat different types of alveolar bone
defects. They have a function to act with osteoconductive or
osteoinductive properties. They can maintain a space and play an
evident role by preventing membrane from collapse in the bone
defect [30,31]. They can also support the flap, facilitate the wound
stability process and enhance the regenerative procedure [32].
Equine bone graft showed a favorable clinical and histological
outcomes and an effective therapy for periodontal guided tissue
and bone regeneration of intrabony defects, especially when
combined with equine resorbable membranes [17,18].
Regeneration of aggressive periodontal defects is considered
as a real challenge. Some earlier studies [33-36,13] used
different graft materials and barrier membranes, either alone or
in combination, to achieve periodontal regeneration. However,
the treatment outcome showed the combination therapy
(GTR membrane + bone graft) as more effective than using
GTR membrane or graft alone. Most of these studies showed a
combination of GTR membrane with either allograft (DFDBA),
xenograft (Bio-Oss), hydroxyapatite, or enamel matrix proteins.
However, in recent years, some evidence [17,18] suggested that
equine bone graft and equine membrane are also capable of
supporting the periodontal regenerative healing capacity. The
present study was therefore planned to evaluate and compare
the efficacy of using equine bio absorbable barrier, either alone
or combined with equine graft, in treating intrabony defects of
aggressive periodontitis.
It has been noticed that the clinical measurements have
a critical role in evaluating regenerative process since they
can provide reliable information regarding probing depth
reduction and clinical attachment level gain. The studied groups
of the present study showed significant improvement of PPD
& CAL parameters when comparing baseline with 18 month
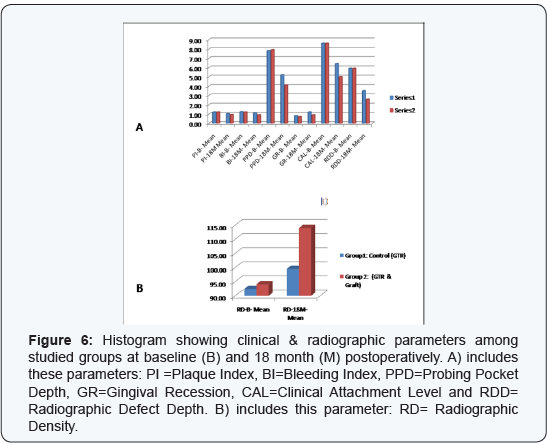
post-operative data (Table 1) & (Figure 6), thus signifying the regenerative role of GTR materials. In this context, Garrett
in (1996) [37] stated that use of GTR membrane enhanced
successfully re-growth of the destroyed periodontium; however,
there are some variations in the clinical predictability, degree
of efficacy, and even the histological outcomes as postulated
by Bartold et al. [38]. In addition, the intergroup comparison
showed more probing depth reduction and greater attachment
level gain in group 2 (the combined therapy) over group 1 (GTR
alone) (Table 1) & (Figure 6), since statistically significant
differences were found at 18 months post-operatively. This
means that the attachment gain complied with the findings
obtained in the previous studies [33-36,12-14]. However, there
was a statistically significant difference found within group 1
when comparing gingival recession parameter from baseline up
to 18 month denoting some gingival recession increase occurred
in this group, whereas no significant difference noticed within
group 2. On the other hand, the comparison between the two
groups did not notice significant difference for gingival recession
(Table 1). In addition, the membrane exposure was occurred in
only one defect site per each group, but controlled with a careful
post-operative care without complication.
The radiographic analysis is one of the valid methods
to demonstrate the effectiveness of regenerative therapies,
since it plays a certain role in determining treatment outcome
through offering a non-invasive method of evaluating the hard
tissue response to therapy. The radiographic analysis of the
present study included linear measurements and bone density
assessment using gray level. The results showed significant
defect resolutions through an evident improvement in defect
depth as well as a gain in bone density in both studied groups,
when comparing baseline data with results of 18 month. Also,
the intergroup comparison showed statistically significant
differences favoring the results of the combined therapy (GTR +
graft) over using GTR alone (Table 1) & (Figure 6). On the other
hand, the changes in alveolar crest height were comparable in
both groups. These results are consistent with the obtained
findings of some previous reports [34-36,13-16,39].
The significant improvement of the combined therapy in
the present study may be explained by the use of bone graft as
defect filler. In this context, some studies [14,40] suggested that
treatment of periodontal intrabony defects with graft materials
lead to significantly greater gain in clinical attachment level
and better defect fill, by promoting osteogenesis and allowing
rapid and quick formation of new bone. Also, it appears that the
graft material has a critical role in preventing collapse of the
membrane and/or flap during initial healing periods, thus can
potentiate regeneration [32].
Regarding the positive findings obtained in the present study
and as a significant point of view, it has been noticed that the ten
involved cases were selected carefully with a complete patient
desire to treat the affected defects, to strictly follow instructions
to maintain oral hygiene and to attend needed follow up visits in
due times with almost a complete compliance. The subjects had
comparable bilateral intrabony defects. Their ages were below
thirty in seven subjects and above thirty in only three patients.
Mobility did not has any worsening in their grades throughout
the whole study interval. Regarding pocket depth reduction,
the number of defects that showed a successful resolution were
four in Group 1 (GTR alone) and seven in group 2 (combined
therapy), whereas the remaining defects (six in group 1 and
three in group 2) still had a severe probing periodontal pocket
depths. These remaining defects ranged in their depths from 5
to 8 mm in group 1 but from 5 to 6 mm in group 2. Regarding
radiographic defect depth resolution and radiographic density
gain, group 2 showed favored significant results, but no defects
showed complete fill radiographically.
Conclusion
In final conclusion, although the present split-mouth clinical
study had some given constraints, the combined therapy of using
graft material (equine bone, Bio-Gen®) with GTR bio absorbable
membrane (equine collagen, Biocollagen®) showed enhanced
and significant clinical outcomes (PPD & CAL) over using GTR
alone. Also, the radiographic assessment that evaluated defect fill
(RDD) and bone density (RD) showed significantly greater results
of the combined therapy. However, the studied groups showed
significant improvement of these parameters when comparing
baseline data with results of 18 month postoperatively within
each group.
Meanwhile, we are planning to make further future long-term
follow up (3 & 5 years) of results of the present study. However,
further studies with larger sample size and different evaluation
methods such as histological assessment may be recommended.
Acknowledgement
The author would like to thank Mss. Afaf El-Ewaidy for her
helpful assistance during the preparation of this manuscript. The
author would also like to forward sincere regards & gratefulness
for the kind great efforts that is done in the radiographic analysis needed for this research by Dr. Hisashi Watanabe, Associate
Professor, Section of Periodontology, Department of Hard
Tissue Engineering, Graduate School, Tokyo Medical and Dental
University, Tokyo, Japan.
Foot Notes
- *Biocollagen®, the product support bone remodelling phase, Lyophilized Equine bioabsorbable collagen membrane, 25 x 25 x 0.2 mm, Bioteck, Torino, Italy.
- †Bio-Gen®, bone tissue of animal Equine origin “xenograft”, deantigenized for total reabsorption, Bio-Gen Mix, Cortical-Spongy, GR. 0.5 size 0.5 – 1 mm, Bioteck, Torino, Italy.
- #Hu-Friedy, Chicago, IL, USA.
- $Cavitron, Dentsply, NY, USA.
- ‡Florida probe, Florida Probe Corporation, Gaineswille, FL, USA.
- %Coe-pak, GC America.
- @Emago Dental Software, Oral Diagnostics Dental Systems, Amsterdam, The Netherlands.
For more Open Access Journals in Juniper Publishers please
click on: https://juniperpublishers.com
For more articles
in Open Access Journal of Dentistry & Oral Health please click on:

Comments
Post a Comment